EphrinB3/EphA4-mediated guidance of ascending and descending spinal tracts
- PMID: 24360544
- PMCID: PMC4030410
- DOI: 10.1016/j.neuron.2013.10.006
EphrinB3/EphA4-mediated guidance of ascending and descending spinal tracts
Abstract
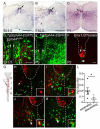
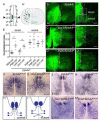
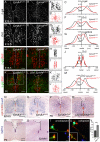
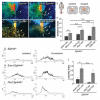
The spinal cord contains many descending and ascending longitudinal tracts whose development appears to be controlled by distinct guidance systems. We identified a population of dorsal spinal neurons marked by coexpression of the transcription factor Zic2 and the guidance receptor EphA4. Zic2+;EphA4+ neurons are surrounded by mechanosensory terminals, suggesting innervation by mechanoreceptor afferents. Their axons form an ipsilateral ascending pathway that develops during embryogenesis and projects within the ventral aspect of the dorsal funiculus, the same location as the descending corticospinal tract (CST), which develops postnatally. Interestingly, the same guidance mechanism, namely, ephrinB3-induced EphA4 forward signaling, is required for the guidance of both ascending and descending axon tracts. Our analysis of conditional EphA4 mutant mice also revealed that the development of the dorsal funiculus occurs independently of EphA4 expression in descending CST axons and is linked to the distribution of Zic2+;EphA4+ spinal neurons and the formation of the ascending pathway.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Zic2-dependent axon midline avoidance controls the formation of major ipsilateral tracts in the CNS.Neuron. 2013 Dec 18;80(6):1392-406. doi: 10.1016/j.neuron.2013.10.007. Neuron. 2013. PMID: 24360543
-
There's more than one way to skin a chimaerin.Neuron. 2007 Sep 6;55(5):681-4. doi: 10.1016/j.neuron.2007.08.015. Neuron. 2007. PMID: 17785174 Free PMC article.
-
Development and reorganization of corticospinal projections in EphA4 deficient mice.J Comp Neurol. 2001 Jul 23;436(2):248-62. J Comp Neurol. 2001. PMID: 11438928
-
The development of mammalian motor systems: the opossum Monodelphis domestica as a model.Brain Res Bull. 2000 Nov 15;53(5):615-26. doi: 10.1016/s0361-9230(00)00395-6. Brain Res Bull. 2000. PMID: 11165797 Review.
-
Physiological, anatomical and genetic identification of CPG neurons in the developing mammalian spinal cord.Prog Neurobiol. 2003 Jul;70(4):347-61. doi: 10.1016/s0301-0082(03)00091-1. Prog Neurobiol. 2003. PMID: 12963092 Review.
Cited by
-
SpinalTRAQ: A novel volumetric cervical spinal cord atlas identifies the corticospinal tract synaptic projectome in healthy and post-stroke mice.bioRxiv [Preprint]. 2024 Oct 10:2024.08.23.609434. doi: 10.1101/2024.08.23.609434. bioRxiv. 2024. Update in: eNeuro. 2025 Sep 2:ENEURO.0276-25.2025. doi: 10.1523/ENEURO.0276-25.2025. PMID: 39416130 Free PMC article. Updated. Preprint.
-
Ephrin-B3 supports glioblastoma growth by inhibiting apoptosis induced by the dependence receptor EphA4.Oncotarget. 2017 Apr 4;8(14):23750-23759. doi: 10.18632/oncotarget.16077. Oncotarget. 2017. PMID: 28423606 Free PMC article.
-
EPHA4 signaling dysregulation links abnormal locomotion and the development of idiopathic scoliosis.Elife. 2025 Jul 15;13:RP95324. doi: 10.7554/eLife.95324. Elife. 2025. PMID: 40662934 Free PMC article.
-
FLRT2 and FLRT3 Cooperate in Maintaining the Tangential Migratory Streams of Cortical Interneurons during Development.J Neurosci. 2021 Sep 1;41(35):7350-7362. doi: 10.1523/JNEUROSCI.0380-20.2021. Epub 2021 Jul 23. J Neurosci. 2021. PMID: 34301831 Free PMC article.
-
Diversity of molecularly defined spinal interneurons engaged in mammalian locomotor pattern generation.J Neurophysiol. 2017 Dec 1;118(6):2956-2974. doi: 10.1152/jn.00322.2017. Epub 2017 Aug 30. J Neurophysiol. 2017. PMID: 28855288 Free PMC article. Review.
References
-
- Bink K, Walch A, Feuchtinger A, Eisenmann H, Hutzler P, Höfler H, Werner M. TO-PRO-3 is an optimal fluorescent dye for nuclear counterstaining in dual-colour FISH on paraffin sections. Histochem Cell Biol. 2001;115:293–299. - PubMed
-
- Coonan JR, Greferath U, Messenger J, Hartley L, Murphy M, Boyd AW, Dottori M, Galea MP, Bartlett PF. Development and reorganization of corticospinal projections in EphA4 deficient mice. J Comp Neurol. 2001;436:248–262. - PubMed
-
- Dottori M, Hartley L, Galea M, Paxinos G, Polizzotto M, Kilpatrick T, Bartlett PF, Murphy M, Köntgen F, Boyd AW. EphA4 (Sek1) receptor tyrosine kinase is required for the development of the corticospinal tract. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13248–13253. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

