Bisphosphonate-functionalized gold nanoparticles for contrast-enhanced X-ray detection of breast microcalcifications
- PMID: 24360718
- PMCID: PMC3956113
- DOI: 10.1016/j.biomaterials.2013.11.077
Bisphosphonate-functionalized gold nanoparticles for contrast-enhanced X-ray detection of breast microcalcifications
Abstract
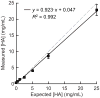
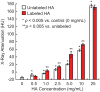
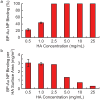
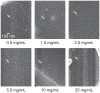
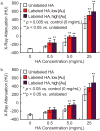
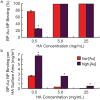
Microcalcifications are one of the most common abnormalities detected by mammography for the diagnosis of breast cancer. However, the detection of microcalcifications and correct diagnosis of breast cancer are limited by the sensitivity and specificity of mammography. Therefore, the objective of this study was to investigate the potential of bisphosphonate-functionalized gold nanoparticles (BP-Au NPs) for contrast-enhanced radiographic detection of breast microcalcifications using two models of breast microcalcifications, which allowed for precise control over levels of hydroxyapatite (HA) mineral within a low attenuating matrix. First, an in vitro imaging phantom was prepared with varying concentrations of HA uniformly dispersed in an agarose hydrogel. The X-ray attenuation of HA-agarose compositions labeled by BP-Au NPs was increased by up to 26 HU compared to unlabeled compositions for HA concentrations ranging from 1 to 10 mg/mL. Second, an ex vivo tissue model was developed to more closely mimic the heterogeneity of breast tissue by injecting varying concentrations of HA in a Matrigel carrier into murine mammary glands. The X-ray attenuation of HA-Matrigel compositions labeled by BP-Au NPs was increased by up to 289 HU compared to unlabeled compositions for HA concentrations ranging from 0.5 to 25 mg/mL, which included an HA concentration (0.5 mg/mL) that was otherwise undetectable by micro-computed tomography. Cumulatively, both models demonstrated the ability of BP-Au NPs to enhance contrast for radiographic detection of microcalcifications, including at a clinically-relevant imaging resolution. Therefore, BP-Au NPs may have potential to improve clinical detection of breast microcalcifications by mammography.
Keywords: Breast cancer; Computed tomography; Contrast agent; Gold nanoparticles; Mammary gland; Microcalcification.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures


References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. - PubMed
-
- Tabar L, Fagerberg CJ, Gad A, Baldetorp L, Holmberg LH, Gröntoft O, Ljungquist U, Lundström B, Månson JC, Eklund G. Reduction in mortality from breast cancer after mass screening with mammography. Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet. 1985;325(8433):829–832. - PubMed
-
- Tabar L, Vitak B, Chen THH, Yen AMF, Cohen A, Tot T, Chiu SYH, Chen SLS, Fann JCY, Rosell J, Fohlin H, Smith RA, Duffy SW. Swedish two-county trial: Impact of mammographic screening on breast cancer mortality during three decades. Radiology. 2011;260(3):658–663. - PubMed
-
- Leborgne R. Diagnosis of tumors of the breast by simple roentgenography; calcifications in carcinomas. Am J Roentgenol Radium Ther. 1951;65(1):1–11. - PubMed
-
- Morgan MP, Cooke MM, McCarthy GM. Microcalcifications associated with breast cancer: An epiphenomenon or biologically significant feature of selected tumors? J Mammary Gland Biol Neoplasia. 2005;10(2):181–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

