Mutations in CSPP1, encoding a core centrosomal protein, cause a range of ciliopathy phenotypes in humans
- PMID: 24360803
- PMCID: PMC3882732
- DOI: 10.1016/j.ajhg.2013.11.010
Mutations in CSPP1, encoding a core centrosomal protein, cause a range of ciliopathy phenotypes in humans
Abstract
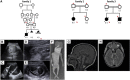
Ciliopathies are characterized by a pattern of multisystem involvement that is consistent with the developmental role of the primary cilium. Within this biological module, mutations in genes that encode components of the cilium and its anchoring structure, the basal body, are the major contributors to both disease causality and modification. However, despite rapid advances in this field, the majority of the genes that drive ciliopathies and the mechanisms that govern the pronounced phenotypic variability of this group of disorders remain poorly understood. Here, we show that mutations in CSPP1, which encodes a core centrosomal protein, are disease causing on the basis of the independent identification of two homozygous truncating mutations in three consanguineous families (one Arab and two Hutterite) affected by variable ciliopathy phenotypes ranging from Joubert syndrome to the more severe Meckel-Gruber syndrome with perinatal lethality and occipital encephalocele. Consistent with the recently described role of CSPP1 in ciliogenesis, we show that mutant fibroblasts from one affected individual have severely impaired ciliogenesis with concomitant defects in sonic hedgehog (SHH) signaling. Our results expand the list of centrosomal proteins implicated in human ciliopathies.
Copyright © 2014 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

