Dominant mutations in GRHL3 cause Van der Woude Syndrome and disrupt oral periderm development
- PMID: 24360809
- PMCID: PMC3882735
- DOI: 10.1016/j.ajhg.2013.11.009
Dominant mutations in GRHL3 cause Van der Woude Syndrome and disrupt oral periderm development
Abstract
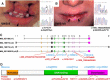
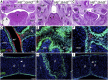
Mutations in interferon regulatory factor 6 (IRF6) account for ∼70% of cases of Van der Woude syndrome (VWS), the most common syndromic form of cleft lip and palate. In 8 of 45 VWS-affected families lacking a mutation in IRF6, we found coding mutations in grainyhead-like 3 (GRHL3). According to a zebrafish-based assay, the disease-associated GRHL3 mutations abrogated periderm development and were consistent with a dominant-negative effect, in contrast to haploinsufficiency seen in most VWS cases caused by IRF6 mutations. In mouse, all embryos lacking Grhl3 exhibited abnormal oral periderm and 17% developed a cleft palate. Analysis of the oral phenotype of double heterozygote (Irf6(+/-);Grhl3(+/-)) murine embryos failed to detect epistasis between the two genes, suggesting that they function in separate but convergent pathways during palatogenesis. Taken together, our data demonstrated that mutations in two genes, IRF6 and GRHL3, can lead to nearly identical phenotypes of orofacial cleft. They supported the hypotheses that both genes are essential for the presence of a functional oral periderm and that failure of this process contributes to VWS.
Copyright © 2014 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Non-random distribution of deleterious mutations in the DNA and protein-binding domains of IRF6 are associated with Van Der Woude syndrome.Mol Genet Genomic Med. 2020 Aug;8(8):e1355. doi: 10.1002/mgg3.1355. Epub 2020 Jun 17. Mol Genet Genomic Med. 2020. PMID: 32558391 Free PMC article. Review.
-
Altered regulation of cell migration in IRF6-mutated orofacial cleft patients-derived primary cells reveals a novel role of Rho GTPases in cleft/lip palate development.Cells Dev. 2021 Jun;166:203674. doi: 10.1016/j.cdev.2021.203674. Epub 2021 Mar 17. Cells Dev. 2021. PMID: 33994351
-
Association study between Van der Woude Syndrome causative gene GRHL3 and nonsyndromic cleft lip with or without cleft palate in a Chinese cohort.Gene. 2016 Aug 15;588(1):69-73. doi: 10.1016/j.gene.2016.04.045. Epub 2016 Apr 26. Gene. 2016. PMID: 27129939
-
IRF6 mutation screening in non-syndromic orofacial clefting: analysis of 1521 families.Clin Genet. 2016 Jul;90(1):28-34. doi: 10.1111/cge.12675. Epub 2015 Oct 1. Clin Genet. 2016. PMID: 26346622 Free PMC article.
-
Toward an orofacial gene regulatory network.Dev Dyn. 2016 Mar;245(3):220-32. doi: 10.1002/dvdy.24341. Epub 2015 Sep 17. Dev Dyn. 2016. PMID: 26332872 Free PMC article. Review.
Cited by
-
Spotlight on the Granules (Grainyhead-Like Proteins) - From an Evolutionary Conserved Controller of Epithelial Trait to Pioneering the Chromatin Landscape.Front Mol Biosci. 2020 Aug 21;7:213. doi: 10.3389/fmolb.2020.00213. eCollection 2020. Front Mol Biosci. 2020. PMID: 32974388 Free PMC article. Review.
-
Non-random distribution of deleterious mutations in the DNA and protein-binding domains of IRF6 are associated with Van Der Woude syndrome.Mol Genet Genomic Med. 2020 Aug;8(8):e1355. doi: 10.1002/mgg3.1355. Epub 2020 Jun 17. Mol Genet Genomic Med. 2020. PMID: 32558391 Free PMC article. Review.
-
Lack of Association between Missense Variants in GRHL3 (rs2486668 and rs545809) and Susceptibility to Non-Syndromic Orofacial Clefts in a Han Chinese Population.PLoS One. 2016 Jul 26;11(7):e0159940. doi: 10.1371/journal.pone.0159940. eCollection 2016. PLoS One. 2016. PMID: 27459192 Free PMC article.
-
Reciprocal interplay between thyroid hormone and microRNA-21 regulates hedgehog pathway-driven skin tumorigenesis.J Clin Invest. 2016 Jun 1;126(6):2308-20. doi: 10.1172/JCI84465. Epub 2016 May 9. J Clin Invest. 2016. PMID: 27159391 Free PMC article.
-
Genome-wide analysis of copy-number variation in humans with cleft lip and/or cleft palate identifies COBLL1, RIC1, and ARHGEF38 as clefting genes.Am J Hum Genet. 2023 Jan 5;110(1):71-91. doi: 10.1016/j.ajhg.2022.11.012. Epub 2022 Dec 8. Am J Hum Genet. 2023. PMID: 36493769 Free PMC article.
References
-
- Mace K.A., Pearson J.C., McGinnis W. An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science. 2005;308:381–385. - PubMed
-
- Ting S.B., Caddy J., Hislop N., Wilanowski T., Auden A., Zhao L.L., Ellis S., Kaur P., Uchida Y., Holleran W.M. A homolog of Drosophila grainy head is essential for epidermal integrity in mice. Science. 2005;308:411–413. - PubMed
-
- Yu Z., Lin K.K., Bhandari A., Spencer J.A., Xu X., Wang N., Lu Z., Gill G.N., Roop D.R., Wertz P., Andersen B. The Grainyhead-like epithelial transactivator Get-1/Grhl3 regulates epidermal terminal differentiation and interacts functionally with LMO4. Dev. Biol. 2006;299:122–136. - PubMed
-
- de la Garza G., Schleiffarth J.R., Dunnwald M., Mankad A., Weirather J.L., Bonde G., Butcher S., Mansour T.A., Kousa Y.A., Fukazawa C.F. Interferon regulatory factor 6 promotes differentiation of the periderm by activating expression of Grainyhead-like 3. J. Invest. Dermatol. 2013;133:68–77. - PMC - PubMed
-
- Sabel J.L., d’Alençon C., O’Brien E.K., Van Otterloo E., Lutz K., Cuykendall T.N., Schutte B.C., Houston D.W., Cornell R.A. Maternal Interferon Regulatory Factor 6 is required for the differentiation of primary superficial epithelia in Danio and Xenopus embryos. Dev. Biol. 2009;325:249–262. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- AR061586/AR/NIAMS NIH HHS/United States
- R37 DE008559/DE/NIDCR NIH HHS/United States
- R01 AR044882/AR/NIAMS NIH HHS/United States
- AR44882/AR/NIAMS NIH HHS/United States
- F31 DE022696/DE/NIDCR NIH HHS/United States
- DE021071/DE/NIDCR NIH HHS/United States
- R01 DE021071/DE/NIDCR NIH HHS/United States
- DE13513/DE/NIDCR NIH HHS/United States
- GM008629/GM/NIGMS NIH HHS/United States
- DE08559/DE/NIDCR NIH HHS/United States
- R01 DE023575/DE/NIDCR NIH HHS/United States
- R01 DE013513/DE/NIDCR NIH HHS/United States
- F31DE022696/DE/NIDCR NIH HHS/United States
- T32 HD060555/HD/NICHD NIH HHS/United States
- R01 DE008559/DE/NIDCR NIH HHS/United States
- R03 AR061586/AR/NIAMS NIH HHS/United States
- T32 GM008629/GM/NIGMS NIH HHS/United States
- U01 DE020057/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

