BDNF mediates adaptive brain and body responses to energetic challenges
- PMID: 24361004
- PMCID: PMC3915771
- DOI: 10.1016/j.tem.2013.10.006
BDNF mediates adaptive brain and body responses to energetic challenges
Abstract
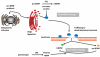
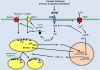
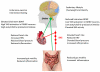
Emerging findings suggest that brain-derived neurotrophic factor (BDNF) serves widespread roles in regulating energy homeostasis by controlling patterns of feeding and physical activity, and by modulating glucose metabolism in peripheral tissues. BDNF mediates the beneficial effects of energetic challenges such as vigorous exercise and fasting on cognition, mood, cardiovascular function, and on peripheral metabolism. By stimulating glucose transport and mitochondrial biogenesis BDNF bolsters cellular bioenergetics and protects neurons against injury and disease. By acting in the brain and periphery, BDNF increases insulin sensitivity and parasympathetic tone. Genetic factors, a 'couch potato' lifestyle, and chronic stress impair BDNF signaling, and this may contribute to the pathogenesis of metabolic syndrome. Novel BDNF-focused interventions are being developed for obesity, diabetes, and neurological disorders.
Keywords: Alzheimer's disease; BDNF; diabetes; exercise; glucocorticoid; insulin resistance; learning and memory; obesity.
Published by Elsevier Ltd.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

