Flow of cortical activity underlying a tactile decision in mice
- PMID: 24361077
- PMCID: PMC3984938
- DOI: 10.1016/j.neuron.2013.10.020
Flow of cortical activity underlying a tactile decision in mice
Abstract
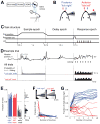
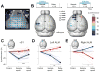
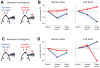
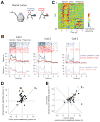
Perceptual decisions involve distributed cortical activity. Does information flow sequentially from one cortical area to another, or do networks of interconnected areas contribute at the same time? Here we delineate when and how activity in specific areas drives a whisker-based decision in mice. A short-term memory component temporally separated tactile "sensation" and "action" (licking). Using optogenetic inhibition (spatial resolution, 2 mm; temporal resolution, 100 ms), we surveyed the neocortex for regions driving behavior during specific behavioral epochs. Barrel cortex was critical for sensation. During the short-term memory, unilateral inhibition of anterior lateral motor cortex biased responses to the ipsilateral side. Consistently, barrel cortex showed stimulus-specific activity during sensation, whereas motor cortex showed choice-specific preparatory activity and movement-related activity, consistent with roles in motor planning and movement. These results suggest serial information flow from sensory to motor areas during perceptual decision making.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment in
-
From perception to action: a spatiotemporal cortical map.Neuron. 2014 Jan 8;81(1):5-8. doi: 10.1016/j.neuron.2013.12.020. Neuron. 2014. PMID: 24411727
References
-
- Afraz SR, Kiani R, Esteky H. Microstimulation of inferotemporal cortex influences face categorization. Nature. 2006;442:692–695. - PubMed
-
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4:S143–156. - PubMed
-
- Armstrong-James M, Fox K, Das-Gupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. J Neurosci. 1992;68:1345–1354. - PubMed
-
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, series B. 1995;57:289–300.
-
- Bisley JW, Zaksas D, Pasternak T. Microstimulation of cortical area MT affects performance on a visual working memory task. Journal of neurophysiology. 2001;85:187–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

