Gluconeogenic signals regulate iron homeostasis via hepcidin in mice
- PMID: 24361124
- PMCID: PMC3989026
- DOI: 10.1053/j.gastro.2013.12.016
Gluconeogenic signals regulate iron homeostasis via hepcidin in mice
Abstract
Background & aims: Hepatic gluconeogenesis provides fuel during starvation, and is abnormally induced in obese individuals or those with diabetes. Common metabolic disorders associated with active gluconeogenesis and insulin resistance (obesity, metabolic syndrome, diabetes, and nonalcoholic fatty liver disease) have been associated with alterations in iron homeostasis that disrupt insulin sensitivity and promote disease progression. We investigated whether gluconeogenic signals directly control Hepcidin, an important regulator of iron homeostasis, in starving mice (a model of persistently activated gluconeogenesis and insulin resistance).
Methods: We investigated hepatic regulation of Hepcidin expression in C57BL/6Crl, 129S2/SvPas, BALB/c, and Creb3l3-/- null mice. Mice were fed a standard, iron-balanced chow diet or an iron-deficient diet for 9 days before death, or for 7 days before a 24- to 48-hour starvation period; liver and spleen tissues then were collected and analyzed by quantitative reverse-transcription polymerase chain reaction and immunoblot analyses. Serum levels of iron, hemoglobin, Hepcidin, and glucose also were measured. We analyzed human hepatoma (HepG2) cells and mouse primary hepatocytes to study transcriptional control of Hamp (the gene that encodes Hepcidin) in response to gluconeogenic stimuli using small interfering RNA, luciferase promoter, and chromatin immunoprecipitation analyses.
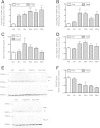
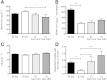
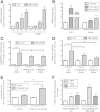
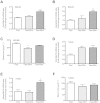
Results: Starvation led to increased transcription of the gene that encodes phosphoenolpyruvate carboxykinase 1 (a protein involved in gluconeogenesis) in livers of mice, increased levels of Hepcidin, and degradation of Ferroportin, compared with nonstarved mice. These changes resulted in hypoferremia and iron retention in liver tissue. Livers of starved mice also had increased levels of Ppargc1a mRNA and Creb3l3 mRNA, which encode a transcriptional co-activator involved in energy metabolism and a liver-specific transcription factor, respectively. Glucagon and a cyclic adenosine monophosphate analog increased promoter activity and transcription of Hamp in cultured liver cells; levels of Hamp were reduced after administration of small interfering RNAs against Ppargc1a and Creb3l3. PPARGC1A and CREB3L3 bound the Hamp promoter to activate its transcription in response to a cyclic adenosine monophosphate analog. Creb3l3-/- mice did not up-regulate Hamp or become hypoferremic during starvation.
Conclusions: We identified a link between glucose and iron homeostasis, showing that Hepcidin is a gluconeogenic sensor in mice during starvation. This response is involved in hepatic metabolic adaptation to increased energy demands; it preserves tissue iron for vital activities during food withdrawal, but can cause excessive iron retention and hypoferremia in disorders with persistently activated gluconeogenesis and insulin resistance.
Keywords: Glucagon; Mouse Model; Peroxisome Proliferator-Activated Receptor-Gamma Co-activator 1-Alpha (PGC1A); cAMP Response Element-Binding Protein-H (CREBH).
Copyright © 2014 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Spiegelman B.M., Heinrich R. Biological control through regulated transcriptional coactivators. Cell. 2004;119:157–167. - PubMed
-
- Cahill G.F., Jr. Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. - PubMed
-
- Muhlenhoff U., Lill R. Biogenesis of iron-sulfur proteins in eukaryotes: a novel task of mitochondria that is inherited from bacteria. Biochim Biophys Acta. 2000;1459:370–382. - PubMed
-
- Bekri S., Gual P., Anty R. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131:788–796. - PubMed
-
- Mendler M.H., Turlin B., Moirand R. Insulin resistance-associated hepatic iron overload. Gastroenterology. 1999;117:1155–1163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

