APOBEC3 multimerization correlates with HIV-1 packaging and restriction activity in living cells
- PMID: 24361275
- PMCID: PMC3977201
- DOI: 10.1016/j.jmb.2013.12.014
APOBEC3 multimerization correlates with HIV-1 packaging and restriction activity in living cells
Abstract
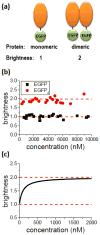
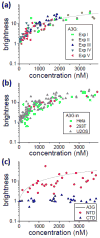
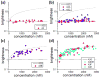
APOBEC3G belongs to a family of DNA cytosine deaminases that are involved in the restriction of a broad number of retroviruses including human immunodeficiency virus type 1 (HIV-1). Prior studies have identified two distinct mechanistic steps in Vif-deficient HIV-1 restriction: packaging into virions and deaminating viral cDNA. APOBEC3A, for example, although highly active, is not packaged and is therefore not restrictive. APOBEC3G, on the other hand, although having weaker enzymatic activity, is packaged into virions and is strongly restrictive. Although a number of studies have described the propensity for APOBEC3 oligomerization, its relevance to HIV-1 restriction remains unclear. Here, we address this problem by examining APOBEC3 oligomerization in living cells using molecular brightness analysis. We find that APOBEC3G forms high-order multimers as a function of protein concentration. In contrast, APOBEC3A, APOBEC3C and APOBEC2 are monomers at all tested concentrations. Among other members of the APOBEC3 family, we show that the multimerization propensities of APOBEC3B, APOBEC3D, APOBEC3F and APOBEC3H (haplotype II) bear more resemblance to APOBEC3G than to APOBEC3A/3C/2. Prior studies have shown that all of these multimerizing APOBEC3 proteins, but not the monomeric family members, have the capacity to package into HIV-1 particles and restrict viral infectivity. This correlation between oligomerization and restriction is further evidenced by two different APOBEC3G mutants, which are each compromised for multimerization, packaging and HIV-1 restriction. Overall, our results imply that multimerization of APOBEC3 proteins may be related to the packaging mechanism and ultimately to virus restriction.
Keywords: APOBEC3G; brightness; fluorescence fluctuation spectroscopy; mobility; molecular mass complex.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
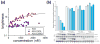
Similar articles
-
Mechanism of Enhanced HIV Restriction by Virion Coencapsidated Cytidine Deaminases APOBEC3F and APOBEC3G.J Virol. 2017 Jan 18;91(3):e02230-16. doi: 10.1128/JVI.02230-16. Print 2017 Feb 1. J Virol. 2017. PMID: 27881650 Free PMC article.
-
Human and rhesus APOBEC3D, APOBEC3F, APOBEC3G, and APOBEC3H demonstrate a conserved capacity to restrict Vif-deficient HIV-1.J Virol. 2011 Nov;85(21):11220-34. doi: 10.1128/JVI.05238-11. Epub 2011 Aug 10. J Virol. 2011. PMID: 21835787 Free PMC article.
-
Stability of APOBEC3F in the Presence of the APOBEC3 Antagonist HIV-1 Vif Increases at the Expense of Co-Expressed APOBEC3H Haplotype I.Viruses. 2023 Feb 7;15(2):463. doi: 10.3390/v15020463. Viruses. 2023. PMID: 36851677 Free PMC article.
-
Interactions of host APOBEC3 restriction factors with HIV-1 in vivo: implications for therapeutics.Expert Rev Mol Med. 2010 Jan 22;12:e4. doi: 10.1017/S1462399409001343. Expert Rev Mol Med. 2010. PMID: 20096141 Free PMC article. Review.
-
Multiple APOBEC3 restriction factors for HIV-1 and one Vif to rule them all.J Mol Biol. 2014 Mar 20;426(6):1220-45. doi: 10.1016/j.jmb.2013.10.033. Epub 2013 Nov 2. J Mol Biol. 2014. PMID: 24189052 Free PMC article. Review.
Cited by
-
Analysis of Arc/Arg3.1 Oligomerization In Vitro and in Living Cells.Int J Mol Sci. 2024 Jun 12;25(12):6454. doi: 10.3390/ijms25126454. Int J Mol Sci. 2024. PMID: 38928159 Free PMC article.
-
Insights into the Structures and Multimeric Status of APOBEC Proteins Involved in Viral Restriction and Other Cellular Functions.Viruses. 2021 Mar 17;13(3):497. doi: 10.3390/v13030497. Viruses. 2021. PMID: 33802945 Free PMC article. Review.
-
APOBEC3: Friend or Foe in Human Papillomavirus Infection and Oncogenesis?Annu Rev Virol. 2022 Sep 29;9(1):375-395. doi: 10.1146/annurev-virology-092920-030354. Epub 2022 Jun 7. Annu Rev Virol. 2022. PMID: 35671565 Free PMC article. Review.
-
Scanning number and brightness yields absolute protein concentrations in live cells: a crucial parameter controlling functional bio-molecular interaction networks.Biophys Rev. 2018 Feb;10(1):87-96. doi: 10.1007/s12551-017-0394-z. Epub 2018 Jan 30. Biophys Rev. 2018. PMID: 29383593 Free PMC article. Review.
-
Foamy Viruses, Bet, and APOBEC3 Restriction.Viruses. 2021 Mar 18;13(3):504. doi: 10.3390/v13030504. Viruses. 2021. PMID: 33803830 Free PMC article. Review.
References
-
- Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

