The solution structure of the regulatory domain of tyrosine hydroxylase
- PMID: 24361276
- PMCID: PMC3951675
- DOI: 10.1016/j.jmb.2013.12.015
The solution structure of the regulatory domain of tyrosine hydroxylase
Abstract
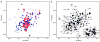
Tyrosine hydroxylase (TyrH) catalyzes the hydroxylation of tyrosine to form 3,4-dihydroxyphenylalanine in the biosynthesis of the catecholamine neurotransmitters. The activity of the enzyme is regulated by phosphorylation of serine residues in a regulatory domain and by binding of catecholamines to the active site. Available structures of TyrH lack the regulatory domain, limiting the understanding of the effect of regulation on structure. We report the use of NMR spectroscopy to analyze the solution structure of the isolated regulatory domain of rat TyrH. The protein is composed of a largely unstructured N-terminal region (residues 1-71) and a well-folded C-terminal portion (residues 72-159). The structure of a truncated version of the regulatory domain containing residues 65-159 has been determined and establishes that it is an ACT domain. The isolated domain is a homodimer in solution, with the structure of each monomer very similar to that of the core of the regulatory domain of phenylalanine hydroxylase. Two TyrH regulatory domain monomers form an ACT domain dimer composed of a sheet of eight strands with four α-helices on one side of the sheet. Backbone dynamic analyses were carried out to characterize the conformational flexibility of TyrH65-159. The results provide molecular details critical for understanding the regulatory mechanism of TyrH.
Keywords: ACT domain; NMR spectroscopy; regulation; solution structure; tyrosine hydroxylase.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures



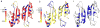
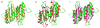

References
-
- Hoffmann GF, Assmann B, Brautigam C, Dionisi-Vici C, Haussler M, de Klerk JB, Naumann M, Steenbergen-Spanjers GC, Strassburg HM, Wevers RA. Tyrosine hydroxylase deficiency causes progressive encephalopathy and dopa-nonresponsive dystonia. Ann Neurol. 2003;54(Suppl 6):S56–S65. - PubMed
-
- Ludecke B, Dworniczak B, Bartholome K. A point mutation in the tyrosine hydroxylase gene associated with Segawa’s syndrome. Hum Genet. 1995;95:123–125. - PubMed
-
- Fitzpatrick PF. Tetrahydropterin-dependent amino acid hydroxylases. Ann Rev Biochem. 1999;68:355–381. - PubMed
-
- Lohse DL, Fitzpatrick PF. Identification of the intersubunit binding region in rat tyrosine hydroxylase. Biochem Biophys Res Commun. 1993;197:1543–1548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

