In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing
- PMID: 24361318
- PMCID: PMC3992176
- DOI: 10.1016/j.jacc.2013.11.023
In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing
Abstract
Objectives: The aim of this study was to test whether silencing of the transcription factor interferon regulatory factor 5 (IRF5) in cardiac macrophages improves infarct healing and attenuates post-myocardial infarction (MI) remodeling.
Background: In healing wounds, the M1 toward M2 macrophage phenotype transition supports resolution of inflammation and tissue repair. Persistence of inflammatory M1 macrophages may derail healing and compromise organ functions. The transcription factor IRF5 up-regulates genes associated with M1 macrophages.
Methods: Here we used nanoparticle-delivered small interfering ribonucleic acid (siRNA) to silence IRF5 in macrophages residing in MIs and in surgically-induced skin wounds in mice.
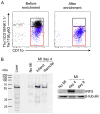
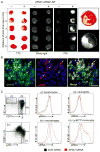
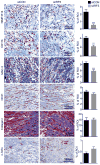
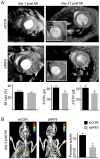
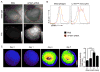
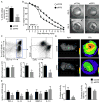
Results: Infarct macrophages expressed high levels of IRF5 during the early inflammatory wound-healing stages (day 4 after coronary ligation), whereas expression of the transcription factor decreased during the resolution of inflammation (day 8). Following in vitro screening, we identified an siRNA sequence that, when delivered by nanoparticles to wound macrophages, efficiently suppressed expression of IRF5 in vivo. Reduction of IRF5 expression, a factor that regulates macrophage polarization, reduced expression of inflammatory M1 macrophage markers, supported resolution of inflammation, accelerated cutaneous and infarct healing, and attenuated development of post-MI heart failure after coronary ligation as measured by protease targeted fluorescence molecular tomography-computed tomography imaging and cardiac magnetic resonance imaging (p < 0.05).
Conclusions: This work identified a new therapeutic avenue to augment resolution of inflammation in healing infarcts by macrophage phenotype manipulation. This therapeutic concept may be used to attenuate post-MI remodeling and heart failure.
Keywords: IRF5; healing; heart failure; macrophage; myocardial infarction.
Copyright © 2014 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures

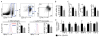
Comment in
-
Could silencing IRF5 improve healing of a myocardial infarct through the reprogramming of the macrophage population?J Am Coll Cardiol. 2014 Apr 22;63(15):1567-8. doi: 10.1016/j.jacc.2013.11.024. Epub 2013 Dec 18. J Am Coll Cardiol. 2014. PMID: 24361313 Free PMC article. No abstract available.
References
-
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. - PubMed
-
- Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–85. - PubMed
-
- Biswas SK, Chittezhath M, Shalova IN, Lim JY. Macrophage polarization and plasticity in health and disease. Immunol Res. 2012;53:11–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

