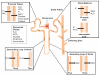
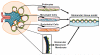
Tissue-engineered kidney disease models
- PMID: 24361391
- PMCID: PMC4019700
- DOI: 10.1016/j.addr.2013.12.002
Tissue-engineered kidney disease models
Abstract
Renal disease represents a major health problem that often results in end-stage renal failure necessitating dialysis and eventually transplantation. Historically these diseases have been studied with patient observation and screening, animal models, and two-dimensional cell culture. In this review, we focus on recent advances in tissue engineered kidney disease models that have the capacity to compensate for the limitations of traditional modalities. The cells and materials utilized to develop these models are discussed and tissue engineered models of polycystic kidney disease, drug-induced nephrotoxicity, and the glomerulus are examined in detail. The application of these models has the potential to direct future disease treatments and preclinical drug development.
Keywords: 3D tissues; Acute kidney injury; Chronic kidney disease; Drug-induced nephrotoxicity; Glomerulus; Microfluidics; Polycystic kidney disease; Renal; Renal cell carcinoma; Renal fibrosis.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures
References
-
- Gibbons MC, Foley MA, Cardinal KO. Thinking inside the box: keeping tissue-engineered constructs in vitro for use as preclinical models. Tissue Eng Part B Rev. 2013;19:14–30. - PubMed
-
- Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, Lilly P, Sanders J, Sipes G, Bracken W, Dorato M, Van DK, Smith P, Berger B, Heller A. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000;32:56–67. - PubMed
-
- Knight A. Systematic reviews of animal experiments demonstrate poor contributions toward human healthcare. Rev Recent Clin. Trials. 2008;3:89–96. - PubMed
-
- Hartung T. Three Rs potential in the development and quality control of pharmaceuticals. ALTEX. 2001;18(Suppl 1):3–13. - PubMed
-
- Hartung T, Bremer S, Casati S, Coecke S, Corvi R, Fortaner S, Gribaldo L, Halder M, Roi AJ, Prieto P, Sabbioni E, Worth A, Zuang V. ECVAM’s response to the changing political environment for alternatives: consequences of the European Union chemicals and cosmetics policies. Altern. Lab Anim. 2003;31:473–481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical