Spinal cord stimulation reduces mechanical hyperalgesia and glial cell activation in animals with neuropathic pain
- PMID: 24361846
- PMCID: PMC4297213
- DOI: 10.1213/ANE.0000000000000047
Spinal cord stimulation reduces mechanical hyperalgesia and glial cell activation in animals with neuropathic pain
Abstract
Background: Spinal cord stimulation (SCS) is commonly used for neuropathic pain; the optimal variables and mechanisms of action are unclear. We tested whether modulation of SCS variables improved analgesia in animals with neuropathic pain by comparing 6-hour vs 30-minute duration and 50%, 75%, or 90% motor threshold (MT) intensity (amplitude). Furthermore, we examined whether maximally effective SCS reduced glial activation in the spinal cord in neuropathic animals.
Methods: Sprague-Dawley rats received the spared nerve injury model and were implanted with an epidural SCS lead. Animals were tested for mechanical withdrawal threshold of the paw before and 2 weeks after spared nerve injury, before and after SCS daily for 4 days, and 1, 4, and 9 days after SCS. Spinal cords were examined for the effects of SCS on glial cell activation.
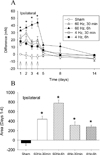
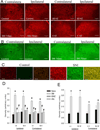
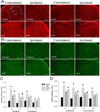
Results: The mechanical withdrawal threshold decreased, and glial immunoreactivity increased 2 weeks after spared nerve injury. For duration, 6-hour SCS significantly increased the mechanical withdrawal threshold when compared with 30-minute SCS or sham SCS; 30-minute SCS was greater than sham SCS. For intensity (amplitude), 90% MT SCS significantly increased the withdrawal threshold when compared with 75% MT SCS, 50% MT SCS, and sham SCS. Both 4 and 60 Hz SCS decreased glial activation (GFAP, MCP-1, and OX-42) in the spinal cord dorsal horn when compared with sham.
Conclusions: Six-hour duration SCS with 90% MT showed the largest increase in mechanical withdrawal threshold, suggesting that the variables of stimulation are important for clinical effectiveness. One potential mechanism for SCS may be to reduce glial activation at the level of the spinal cord.
Conflict of interest statement
Figures

References
-
- Barchini J, Tchachaghian S, Shamaa F, Jabbur SJ, Meyerson BA, Song Z, Linderoth B, Saade NE. Spinal segmental and supraspinal mechanisms underlying the pain-relieving effects of spinal cord stimulation: an experimental study in a rat model of neuropathy. Neuroscience. 2012;215:196–208. - PubMed
-
- Casamenti F, Prosperi C, Scali C, Giovannelli L, Colivicchi MA, Faussone-Pellegrini MS, Pepeu G. Interleukin-1beta activates forebrain glial cells and increases nitric oxide production and cortical glutamate and GABA release in vivo: implications for Alzheimer’s disease. Neuroscience. 1999;91:831–842. - PubMed
-
- Chen T, Willoughby KA, Ellis EF. Group I metabotropic receptor antagonism blocks depletion of calcium stores and reduces potentiated capacitative calcium entry in strain-injured neurons and astrocytes. J Neurotrauma. 2004;21:271–281. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

