The anaphase promoting complex regulates yeast lifespan and rDNA stability by targeting Fob1 for degradation
- PMID: 24361936
- PMCID: PMC3948801
- DOI: 10.1534/genetics.113.158949
The anaphase promoting complex regulates yeast lifespan and rDNA stability by targeting Fob1 for degradation
Abstract
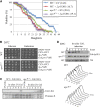
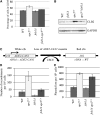
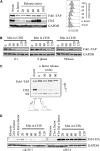
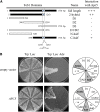
Genomic stability, stress response, and nutrient signaling all play critical, evolutionarily conserved roles in lifespan determination. However, the molecular mechanisms coordinating these processes with longevity remain unresolved. Here we investigate the involvement of the yeast anaphase promoting complex (APC) in longevity. The APC governs passage through M and G1 via ubiquitin-dependent targeting of substrate proteins and is associated with cancer and premature aging when defective. Our two-hybrid screen utilizing Apc5 as bait recovered the lifespan determinant Fob1 as prey. Fob1 is unstable specifically in G1, cycles throughout the cell cycle in a manner similar to Clb2 (an APC target), and is stabilized in APC (apc5(CA)) and proteasome (rpn10) mutants. Deletion of FOB1 increased replicative lifespan (RLS) in wild type (WT), apc5(CA), and apc10 cells, and suppressed apc5(CA) cell cycle progression and rDNA recombination defects. Alternatively, increased FOB1 expression decreased RLS in WT cells, but did not reduce the already short apc5(CA) RLS, suggesting an epistatic interaction between apc5(CA) and fob1. Mutation to a putative L-Box (Fob1(E420V)), a Destruction Box-like motif, abolished Fob1 modifications, stabilized the protein, and increased rDNA recombination. Our work provides a mechanistic role played by the APC to promote replicative longevity and genomic stability in yeast.
Keywords: Fob1; Saccharomyces cerevisiae; anaphase promoting complex; replicative lifespan; two-hybrid screen.
Figures




Similar articles
-
The Saccharomyces cerevisiae anaphase-promoting complex interacts with multiple histone-modifying enzymes to regulate cell cycle progression.Eukaryot Cell. 2010 Oct;9(10):1418-31. doi: 10.1128/EC.00097-10. Epub 2010 Aug 13. Eukaryot Cell. 2010. PMID: 20709786 Free PMC article.
-
The yeast forkhead transcription factors fkh1 and fkh2 regulate lifespan and stress response together with the anaphase-promoting complex.PLoS Genet. 2012;8(3):e1002583. doi: 10.1371/journal.pgen.1002583. Epub 2012 Mar 15. PLoS Genet. 2012. PMID: 22438832 Free PMC article.
-
Contribution of CAF-I to anaphase-promoting-complex-mediated mitotic chromatin assembly in Saccharomyces cerevisiae.Eukaryot Cell. 2005 Apr;4(4):673-84. doi: 10.1128/EC.4.4.673-684.2005. Eukaryot Cell. 2005. PMID: 15821127 Free PMC article.
-
Activating the Anaphase Promoting Complex to Enhance Genomic Stability and Prolong Lifespan.Int J Mol Sci. 2018 Jun 27;19(7):1888. doi: 10.3390/ijms19071888. Int J Mol Sci. 2018. PMID: 29954095 Free PMC article. Review.
-
Altered states: programmed proteolysis and the budding yeast cell cycle.Curr Opin Microbiol. 1999 Dec;2(6):610-7. doi: 10.1016/s1369-5274(99)00030-2. Curr Opin Microbiol. 1999. PMID: 10607629 Review.
Cited by
-
A Genome Scale Screen for Mutants with Delayed Exit from Mitosis: Ire1-Independent Induction of Autophagy Integrates ER Homeostasis into Mitotic Lifespan.PLoS Genet. 2015 Aug 6;11(8):e1005429. doi: 10.1371/journal.pgen.1005429. eCollection 2015 Aug. PLoS Genet. 2015. PMID: 26247883 Free PMC article.
-
The role of Anaphase Promoting Complex activation, inhibition and substrates in cancer development and progression.Aging (Albany NY). 2020 Aug 15;12(15):15818-15855. doi: 10.18632/aging.103792. Epub 2020 Aug 15. Aging (Albany NY). 2020. PMID: 32805721 Free PMC article. Review.
-
The Anaphase-Promoting Complex/Cyclosome Is a Cellular Ageing Regulator.Int J Mol Sci. 2022 Dec 5;23(23):15327. doi: 10.3390/ijms232315327. Int J Mol Sci. 2022. PMID: 36499653 Free PMC article. Review.
-
Using Budding Yeast to Identify Molecules That Block Cancer Cell 'Mitotic Slippage' Only in the Presence of Mitotic Poisons.Int J Mol Sci. 2021 Jul 26;22(15):7985. doi: 10.3390/ijms22157985. Int J Mol Sci. 2021. PMID: 34360748 Free PMC article. Review.
-
RPS12 and UBC4 Are Related to Senescence Signal Production in the Ribosomal RNA Gene Cluster.Mol Cell Biol. 2022 May 19;42(5):e0002822. doi: 10.1128/mcb.00028-22. Epub 2022 Apr 6. Mol Cell Biol. 2022. PMID: 35384721 Free PMC article.
References
-
- Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., et al. , 1995. Current Protocols in Molecular Biology, John Wiley & Sons, New York.
-
- Baker D. J., Jeganathan K. B., Cameron J. D., Thompson M., Juneja S., et al. , 2004. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat. Genet. 36: 744–749. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

