Vanadium exposure induces olfactory dysfunction in an animal model of metal neurotoxicity
- PMID: 24362016
- PMCID: PMC4062607
- DOI: 10.1016/j.neuro.2013.12.004
Vanadium exposure induces olfactory dysfunction in an animal model of metal neurotoxicity
Abstract
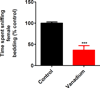
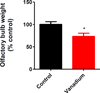
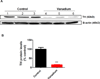
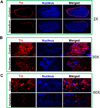
Epidemiological evidence indicates chronic environmental exposure to transition metals may play a role in chronic neurodegenerative conditions such as Parkinson's disease (PD). Chronic inhalation exposure to welding fumes containing metal mixtures may be associated with development of PD. A significant amount of vanadium is present in welding fumes, as vanadium pentoxide (V2O5), and incorporation of vanadium in the production of high strength steel has become more common. Despite the increased vanadium use in recent years, the neurotoxicological effects of this metal are not well characterized. Recently, we demonstrated that V2O5 induces dopaminergic neurotoxicity via protein kinase C delta (PKCδ)-dependent oxidative signaling mechanisms in dopaminergic neuronal cells. Since anosmia (inability to perceive odors) and non-motor deficits are considered to be early symptoms of neurological diseases, in the present study, we examined the effect of V2O5 on the olfactory bulb in animal models. To mimic the inhalation exposure, we intranasally administered C57 black mice a low-dose of 182μg of V2O5 three times a week for one month, and behavioral, neurochemical and biochemical studies were performed. Our results revealed a significant decrease in olfactory bulb weights, tyrosine hydroxylase (TH) levels, levels of dopamine (DA) and its metabolite, 3,4-dihydroxyphenylacetic acid (DOPAC) and increases in astroglia of the glomerular layer of the olfactory bulb in the treatment groups relative to vehicle controls. Neurochemical changes were accompanied by impaired olfaction and locomotion. These findings suggest that nasal exposure to V2O5 adversely affects olfactory bulbs, resulting in neurobehavioral and neurochemical impairments. These results expand our understanding of vanadium neurotoxicity in environmentally-linked neurological conditions.
Keywords: Neurotoxicity; Non-motor symptoms; Olfactory system; Parkinson's disease; Risk assessment; Vanadium.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


Similar articles
-
Manganese and Vanadium Co-Exposure Induces Severe Neurotoxicity in the Olfactory System: Relevance to Metal-Induced Parkinsonism.Int J Mol Sci. 2024 May 13;25(10):5285. doi: 10.3390/ijms25105285. Int J Mol Sci. 2024. PMID: 38791326 Free PMC article.
-
Vanadium induces dopaminergic neurotoxicity via protein kinase Cdelta dependent oxidative signaling mechanisms: relevance to etiopathogenesis of Parkinson's disease.Toxicol Appl Pharmacol. 2009 Oct 15;240(2):273-85. doi: 10.1016/j.taap.2009.07.025. Epub 2009 Jul 29. Toxicol Appl Pharmacol. 2009. PMID: 19646462 Free PMC article.
-
Functional and morphological olfactory bulb modifications in mice after vanadium inhalation.Toxicol Pathol. 2015 Feb;43(2):282-91. doi: 10.1177/0192623314548668. Epub 2014 Dec 9. Toxicol Pathol. 2015. PMID: 25492423
-
Trends in vanadium neurotoxicity.Brain Res Bull. 2019 Feb;145:75-80. doi: 10.1016/j.brainresbull.2018.03.010. Epub 2018 Mar 22. Brain Res Bull. 2019. PMID: 29577939 Review.
-
The intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a new rodent model to test palliative and neuroprotective agents for Parkinson's disease.Curr Pharm Des. 2011;17(5):489-507. doi: 10.2174/138161211795164095. Curr Pharm Des. 2011. PMID: 21375482 Review.
Cited by
-
Characterization of nonmotor behavioral impairments and their neurochemical mechanisms in the MitoPark mouse model of progressive neurodegeneration in Parkinson's disease.Exp Neurol. 2021 Jul;341:113716. doi: 10.1016/j.expneurol.2021.113716. Epub 2021 Apr 8. Exp Neurol. 2021. PMID: 33839143 Free PMC article.
-
Establishment of nasal and olfactory epithelium organoids for unveiling mechanism of tissue regeneration and pathogenesis of nasal diseases.Cell Mol Life Sci. 2025 Jan 3;82(1):33. doi: 10.1007/s00018-024-05557-w. Cell Mol Life Sci. 2025. PMID: 39751829 Free PMC article. Review.
-
Impact of Environmental Risk Factors on Mitochondrial Dysfunction, Neuroinflammation, Protein Misfolding, and Oxidative Stress in the Etiopathogenesis of Parkinson's Disease.Int J Mol Sci. 2022 Sep 16;23(18):10808. doi: 10.3390/ijms231810808. Int J Mol Sci. 2022. PMID: 36142718 Free PMC article. Review.
-
Neuroprotective effect of carnosine in the olfactory bulb after vanadium inhalation in a mouse model.Int J Exp Pathol. 2018 Aug;99(4):180-188. doi: 10.1111/iep.12285. Epub 2018 Sep 9. Int J Exp Pathol. 2018. PMID: 30198103 Free PMC article.
-
Epigallocatechin Gallate Has a Neurorescue Effect in a Mouse Model of Parkinson Disease.J Nutr. 2017 Oct;147(10):1926-1931. doi: 10.3945/jn.117.255034. Epub 2017 Aug 23. J Nutr. 2017. PMID: 28835392 Free PMC article.
References
-
- Adachi A, Asai K, Koyama Y, Matsumoto Y, Kobayashi T. Vanadium content of cigarettes. Bull Environ Contam Toxicol. 1998;61:276–280. - PubMed
-
- Afeseh Ngwa H, Kanthasamy A, Anantharam V, Song C, Witte T, Houk R, Kanthasamy AG. Vanadium induces dopaminergic neurotoxicity via protein kinase Cdelta dependent oxidative signaling mechanisms: relevance to etiopathogenesis of Parkinson's disease. Toxicol Appl Pharmacol. 2009;240:273–285. - PMC - PubMed
-
- Allam MF, Del Castillo AS, Navajas RF. Parkinson's disease risk factors: genetic, environmental, or both? Neurol Res. 2005;27:206–208. - PubMed
-
- Amorim FA, Welz B, Costa AC, Lepri FG, Vale MG, Ferreira SL. Determination of vanadium in petroleum and petroleum products using atomic spectrometric techniques. Talanta. 2007;72:349–359. - PubMed
-
- Anglade P, Vyas S, Hirsch EC, Agid Y. Apoptosis in dopaminergic neurons of the human substantia nigra during normal aging. Histol Histopathol. 1997;12:603–610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

