Structural insights into the epimerization of β-1,4-linked oligosaccharides catalyzed by cellobiose 2-epimerase, the sole enzyme epimerizing non-anomeric hydroxyl groups of unmodified sugars
- PMID: 24362032
- PMCID: PMC3916543
- DOI: 10.1074/jbc.M113.531251
Structural insights into the epimerization of β-1,4-linked oligosaccharides catalyzed by cellobiose 2-epimerase, the sole enzyme epimerizing non-anomeric hydroxyl groups of unmodified sugars
Abstract
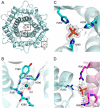
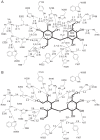
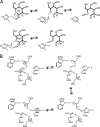
Cellobiose 2-epimerase (CE) reversibly converts d-glucose residues into d-mannose residues at the reducing end of unmodified β1,4-linked oligosaccharides, including β-1,4-mannobiose, cellobiose, and lactose. CE is responsible for conversion of β1,4-mannobiose to 4-O-β-d-mannosyl-d-glucose in mannan metabolism. However, the detailed catalytic mechanism of CE is unclear due to the lack of structural data in complex with ligands. We determined the crystal structures of halothermophile Rhodothermus marinus CE (RmCE) in complex with substrates/products or intermediate analogs, and its apo form. The structures in complex with the substrates/products indicated that the residues in the β5-β6 loop as well as those in the inner six helices form the catalytic site. Trp-322 and Trp-385 interact with reducing and non-reducing end parts of these ligands, respectively, by stacking interactions. The architecture of the catalytic site also provided insights into the mechanism of reversible epimerization. His-259 abstracts the H2 proton of the d-mannose residue at the reducing end, and consistently forms the cis-enediol intermediate by facilitated depolarization of the 2-OH group mediated by hydrogen bonding interaction with His-200. His-390 subsequently donates the proton to the C2 atom of the intermediate to form a d-glucose residue. The reverse reaction is mediated by these three histidines with the inverse roles of acid/base catalysts. The conformation of cellobiitol demonstrated that the deprotonation/reprotonation step is coupled with rotation of the C2-C3 bond of the open form of the ligand. Moreover, it is postulated that His-390 is closely related to ring opening/closure by transferring a proton between the O5 and O1 atoms of the ligand.
Keywords: AGE Superfamily; Bacterial Metabolism; Carbohydrate-binding Protein; Cellobiitol; Cellobiose 2-Epimerase; Crystal Structure; Enzyme Mechanisms; Epimerization; Glycobiology.
Figures


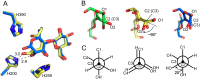


References
-
- Tyler T. R., Leatherwood J. M. (1967) Epimerization of disaccharides by enzyme preparations from Ruminococcus albus. Arch. Biochem. Biophys. 119, 363–367 - PubMed
-
- Senoura T., Ito S., Taguchi H., Higa M., Hamada S., Matsui H., Ozawa T., Jin S., Watanabe J., Wasaki J., Ito S. (2011) New microbial mannan catabolic pathway that involves a novel mannosylglucose phosphorylase. Biochem. Biophys. Res. Commun. 408, 701–706 - PubMed
-
- Kawahara R., Saburi W., Odaka R., Taguchi H., Ito S., Mori H., Matsui H. (2012) Metabolic mechanism of mannan in ruminal bacterium, Ruminococcuss albus, involving two mannoside phosphorylases and cellobiose 2-epimerase. Discovery of a new carbohydrate phosphorylase, β-1,4-mannooligosaccharide phosphorylase. J. Biol. Chem. 287, 42389–42399 - PMC - PubMed
-
- Ito S., Taguchi H., Hamada S., Kawauchi S., Ito H., Senoura T., Watanabe J., Nishimukai M., Ito S., Matsui H. (2008) Enzymatic properties of cellobiose 2-epimerase from Ruminococcus albus and the synthesis of rare oligosaccharides by the enzyme. Appl. Microbiol. Biotechnol. 79, 433–441 - PubMed
-
- Watanabe J., Nishimukai M., Taguchi H., Senoura T., Hamada S., Matsui H., Yamamoto T., Wasaki J., Hara H., Ito S. (2008) Prebiotic properties of epilactose. J. Dairy Sci. 91, 4518–4526 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

