ATPγS competes with ATP for binding at Domain 1 but not Domain 2 during ClpA catalyzed polypeptide translocation
- PMID: 24362308
- PMCID: PMC3966025
- DOI: 10.1016/j.bpc.2013.11.002
ATPγS competes with ATP for binding at Domain 1 but not Domain 2 during ClpA catalyzed polypeptide translocation
Abstract
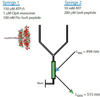
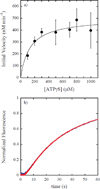
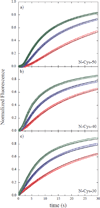
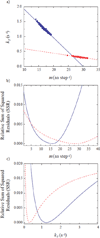
ClpAP is an ATP-dependent protease that assembles through the association of hexameric rings of ClpA with the cylindrically-shaped protease ClpP. ClpA contains two nucleotide binding domains, termed Domain 1 (D1) or 2 (D2). We have proposed that D1 or D2 limits the rate of ClpA catalyzed polypeptide translocation when ClpP is either absent or present, respectively. Here we show that the rate of ClpA catalyzed polypeptide translocation depends on [ATPγS] in the absence of ClpP, but not in the presence of ClpP. We observe that ATPγS non-cooperatively binds to ClpA during polypeptide translocation with an apparent affinity of ~6 μM, but that introduction of ClpP shifts this affinity such that translocation is not affected. Interpreting these data with our proposed model for translocation catalyzed by ClpA vs. ClpAP suggests that ATPγS competes for binding at D1 but not at D2.
Keywords: AAA+ motor proteins; ATP dependent proteases; Pre-steady-state kinetics; Protein unfoldases; Steady-state kinetics.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
E. coli ClpA catalyzed polypeptide translocation is allosterically controlled by the protease ClpP.J Mol Biol. 2013 Aug 9;425(15):2795-812. doi: 10.1016/j.jmb.2013.04.019. Epub 2013 Apr 29. J Mol Biol. 2013. PMID: 23639359 Free PMC article.
-
ClpP hydrolyzes a protein substrate processively in the absence of the ClpA ATPase: mechanistic studies of ATP-independent proteolysis.Biochemistry. 2008 Nov 4;47(44):11536-46. doi: 10.1021/bi801101p. Epub 2008 Oct 8. Biochemistry. 2008. PMID: 18839965
-
Molecular mechanism of polypeptide translocation catalyzed by the Escherichia coli ClpA protein translocase.J Mol Biol. 2010 Jun 25;399(5):665-79. doi: 10.1016/j.jmb.2010.03.061. Epub 2010 Apr 7. J Mol Biol. 2010. PMID: 20380838
-
ClpP: a structurally dynamic protease regulated by AAA+ proteins.J Struct Biol. 2012 Aug;179(2):202-10. doi: 10.1016/j.jsb.2012.05.003. Epub 2012 May 14. J Struct Biol. 2012. PMID: 22595189 Review.
-
Protease Ti (Clp), a multi-component ATP-dependent protease in Escherichia coli.Biol Chem. 1996 Sep;377(9):549-54. Biol Chem. 1996. PMID: 9067252 Review.
Cited by
-
AAA+ protease-adaptor structures reveal altered conformations and ring specialization.Nat Struct Mol Biol. 2022 Nov;29(11):1068-1079. doi: 10.1038/s41594-022-00850-3. Epub 2022 Nov 3. Nat Struct Mol Biol. 2022. PMID: 36329286 Free PMC article.
-
Fundamental Characteristics of AAA+ Protein Family Structure and Function.Archaea. 2016 Sep 14;2016:9294307. doi: 10.1155/2016/9294307. eCollection 2016. Archaea. 2016. PMID: 27703410 Free PMC article. Review.
-
Conformational plasticity of the ClpAP AAA+ protease couples protein unfolding and proteolysis.Nat Struct Mol Biol. 2020 May;27(5):406-416. doi: 10.1038/s41594-020-0409-5. Epub 2020 Apr 20. Nat Struct Mol Biol. 2020. PMID: 32313240 Free PMC article.
-
Reversible Kinetics in Multi-nucleotide Addition Catalyzed by S. cerevisiae RNA polymerase II Reveal Slow Pyrophosphate Release.J Mol Biol. 2024 Jun 15;436(12):168606. doi: 10.1016/j.jmb.2024.168606. Epub 2024 May 8. J Mol Biol. 2024. PMID: 38729258 Free PMC article.
-
Comparative Analysis of the Structure and Function of AAA+ Motors ClpA, ClpB, and Hsp104: Common Threads and Disparate Functions.Front Mol Biosci. 2017 Aug 3;4:54. doi: 10.3389/fmolb.2017.00054. eCollection 2017. Front Mol Biosci. 2017. PMID: 28824920 Free PMC article. Review.
References
-
- Alberts B. The cell as a collection of protein machines: preparing the next generation of molecular biologists. Cell. 1998;92:291–294. - PubMed
-
- Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. - PubMed
-
- Karzai AW, Roche ED, Sauer RT. The SsrA-SmpB system for protein tagging, directed degradation and ribosome rescue. Nat Struct Biol. 2000;7:449–455. - PubMed
-
- Gottesman S. Proteases and their targets in Escherichia coli. Annu Rev Genet. 1996;30:465–506. - PubMed
-
- Sauer RT, Baker TA. AAA+ Proteases: ATP-Fueled Machines of Protein Destruction. Annual review of biochemistry. 2011;80:587–612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

