Administration of deoxyribonucleosides or inhibition of their catabolism as a pharmacological approach for mitochondrial DNA depletion syndrome
- PMID: 24362886
- PMCID: PMC6281351
- DOI: 10.1093/hmg/ddt641
Administration of deoxyribonucleosides or inhibition of their catabolism as a pharmacological approach for mitochondrial DNA depletion syndrome
Abstract
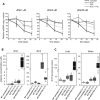
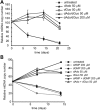
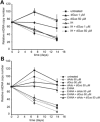
Mitochondrial DNA (mtDNA) depletion syndrome (MDS) is characterized by a reduction in mtDNA copy number and consequent mitochondrial dysfunction in affected tissues. A subgroup of MDS is caused by mutations in genes that disrupt deoxyribonucleotide metabolism, which ultimately leads to limited availability of one or several deoxyribonucleoside triphosphates (dNTPs), and subsequent mtDNA depletion. Here, using in vitro experimental approaches (primary cell culture of deoxyguanosine kinase-deficient cells and thymidine-induced mtDNA depletion in culture as a model of mitochondrial neurogastrointestinal encephalomyopathy, MNGIE), we show that supplements of those deoxyribonucleosides (dNs) involved in each biochemical defect (deoxyguanosine or deoxycytidine, dCtd) prevents mtDNA copy number reduction. Similar effects can be obtained by specific inhibition of dN catabolism using tetrahydrouridine (THU; inhibitor of cytidine deaminase) or immucillin H (inhibitor of purine nucleoside phosphorylase). In addition, using an MNGIE animal model, we provide evidence that mitochondrial dNTP content can be modulated in vivo by systemic administration of dCtd or THU. In spite of the severity associated with diseases due to defects in mtDNA replication, there are currently no effective therapeutic options available. Only in the case of MNGIE, allogeneic hematopoietic stem cell transplantation has proven efficient as a long-term therapeutic strategy. We propose increasing cellular availability of the deficient dNTP precursor by direct administration of the dN or inhibition of its catabolism, as a potential treatment for mtDNA depletion syndrome caused by defects in dNTP metabolism.
Figures
References
-
- Bourdon A., Minai L., Serre V., Jais J.P., Sarzi E., Aubert S., Chretien D., de Lonlay P., Paquis-Flucklinger V., Arakawa H., et al. Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nat. Genet. 2007;39:776–780. - PubMed
-
- Mandel H., Szargel R., Labay V., Elpeleg O., Saada A., Shalata A., Anbinder Y., Berkowitz D., Hartman C., Barak M., et al. The deoxyguanosine kinase gene is mutated in individuals with depleted hepatocerebral mitochondrial DNA. Nat. Genet. 2001;29:337–341. - PubMed
-
- Saada A., Shaag A., Mandel H., Nevo Y., Eriksson S., Elpeleg O. Mutant mitochondrial thymidine kinase in mitochondrial DNA depletion myopathy. Nat. Genet. 2001;29:342–344. - PubMed
-
- Nishino I., Spinazzola A., Hirano M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science. 1999;283:689–692. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

