Daclizumab for relapsing remitting multiple sclerosis
- PMID: 24363032
- PMCID: PMC11491876
- DOI: 10.1002/14651858.CD008127.pub4
Daclizumab for relapsing remitting multiple sclerosis
Abstract
Background: Monoclonal antibodies such as daclizumab could be a possible alternative immunotherapy to interferon beta treatment in people with multiple sclerosis (MS). It blocks the interleukin-2 receptor alpha subunit (CD25), and seems to be beneficial to patients with relapsing remitting multiple sclerosis (RRMS) in clinical and magnetic resonance imaging (MRI) measures of outcomes.This is an update of a Cochrane review first published in 2010, and previously updated in 2012.
Objectives: To assess the safety of daclizumab and its efficacy to prevent clinical worsening in patients with RRMS.
Search methods: The Trials Search Co-ordinator searched the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group Specialised Register (17 May 2013). We handsearched the references quoted in the identified trials and reports (May 2013) from the most important neurological associations and MS societies. We contacted researchers participating in trials on daclizumab.
Selection criteria: All randomised controlled clinical trials (RCTs) evaluating daclizumab, alone or combined with other treatments versus placebo, or any other treatment for patients with RRMS.
Data collection and analysis: Two review authors independently assessed references retrieved for possible inclusion, extracted eligible data, cross-checked the data for accuracy and assessed the methodological quality. We resolved any disagreements by consensus among review authors.
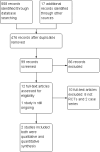
Main results: We included two trials with 851 patients that evaluated the efficacy and safety of daclizumab versus placebo for RRMS. We judged them to be at low risk of bias. Due to different time point evaluations and available data on primary studies, we were unable to undertake a meta-analysis. At 24 weeks, the median change was 0 (range -2 to 3) in the interferon beta and placebo group, 0 (-2 to 4) in the interferon beta and low-dose daclizumab group and 0 (-2 to 2) in the interferon beta and high-dose daclizumab group in 230 participants. The proportion of patients who had new clinical relapses were the following: 16 patients (21%) in the interferon beta and high-dose daclizumab group, 19 (24%) in the interferon beta and low-dose daclizumab group and 19 (25%) in the interferon beta and placebo group had relapses (P value = 0.87). At 52 weeks, the changes in Expanded Disability Status Scale (EDSS) from baseline was 0.09 ± 0.71 in placebo group, -0.08 ± 0.52 in low-dose daclizumab group and 0.05 ± 0.61 in high-dose daclizumab group in 621 participants. There was a significant difference between placebo and low-dose daclizumab groups (P value = 0.01), but no significant difference between placebo and high-dose daclizumab groups (P value = 0.49). The proportion of patients with new relapsing MS was significantly reduced in both daclizumab groups (19% in low-dose daclizumab group, 20% in high-dose daclizumab group) compared with placebo group (36%) (P value < 0.0001 and P value = 0.00032, respectively). There was no increased number of patients in any adverse events (risk ratio (RR) 0.98, 95% confidence interval (CI) 0.89 to 1.07) or serious adverse events in daclizumab groups compared with placebo (RR 1.15, 95% CI 0.29 to 4.54). Infections were the most frequent adverse events in treated participants and were resolved with standard therapies. One trial was still ongoing.
Authors' conclusions: There was insufficient evidence to determine whether daclizumab was more effective than placebo in patients affected by RRMS in terms of clinical and MRI measures of outcomes. Daclizumab appeared to be relatively well tolerated. Infections were the most frequent adverse events, and were resolved with standard therapies.
Conflict of interest statement
None known.
Figures




Update of
-
Daclizumab for relapsing remitting multiple sclerosis.Cochrane Database Syst Rev. 2012 Apr 18;(4):CD008127. doi: 10.1002/14651858.CD008127.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2013 Dec 23;(12):CD008127. doi: 10.1002/14651858.CD008127.pub4. PMID: 22513956 Updated.
References
References to studies included in this review
Gold 2013 {published data only}
-
- Gold R, Giovannoni G, Selmaj K, Havrdova E, Montalban X, Radue EW, et al. Daclizumab high‐yield process in relapsing‐remitting multiple sclerosis (SELECT): a randomised, double‐blind, placebo‐controlled trial. Lancet 2013;381:2167‐75. - PubMed
Wynn 2010 {published data only}
-
- Wynn D, Kaufman M, Montalban X, Vollmer T, Simon J, Elkins J, et al. Daclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomised, double‐blind, placebo‐controlled, add‐on trial with interferon beta. Lancet Neurology 2010;9(4):381‐90. - PubMed
References to studies excluded from this review
Ali 2009 {published data only}
-
- Ali EN, Healy BC, Stazzone LA, Brown BA, Weiner HL, Khoury SJ. Daclizumab in treatment of multiple sclerosis patients. Multiple Sclerosis 2009;15(2):272‐4. - PubMed
Bielekova 2004 {published data only}
-
- Bielekova B, Richert N, Howard T, Blevins G, Markovic‐Plese S, McCartin J, et al. Humanized anti‐CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon beta. Proceedings of the National Academy of Sciences of the United States of America 2004;101(23):8705‐8. - PMC - PubMed
Bielekova 2006 {published data only}
-
- Bielekova B, Catalfamo M, Scrivner SR, Packer A, Cerna M, Waldmann TA, et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL‐2Rα‐targeted therapy (daclizumab) in multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America 2006;103(15):5941‐6. - PMC - PubMed
Bielekova 2009 {published data only}
Bielekova 2011 {published data only}
Borges 2013 {published data only}
Oh 2009 {published data only}
Rose 2004 {published data only}
-
- Rose JM, Watt HE, White AT, Carlson NG. Treatment of multiple sclerosis with an anti‐interleukin‐2 receptor monoclonal antibody. Annals of Neurology 2004;56(6):864‐7. - PubMed
Rose 2007 {published data only}
-
- Rose JW, Burns JB, Bjorklund J, Klein J, Watt HE, Carlson NG. Daclizumab phase II trial in relapsing and remitting multiple sclerosis: MRI and clinical results. Neurology 2007;69(8):785‐9. - PubMed
Yeh 2011 {published data only}
-
- Yeh EA, Waubant E, Krupp LB, Ness J, Chitnis T, Kuntz N, et al. Multiple sclerosis therapies in pediatric patients with refractory multiple sclerosis. Archives of Neurology 2011;68(4):437‐44. - PubMed
References to ongoing studies
NCT01064401 {unpublished data only}
-
- NCT01064401. Efficacy and safety of daclizumab high yield process versus interferon beta 1a in patients with relapsing remitting multiple sclerosis. www.clinicaltrials.gov/ct2/show/NCT01064401 (accessed 11 December 2013).
Additional references
Caligiuri 1993
Compston 2002
-
- Compston A, Coles A. Multiple sclerosis. Lancet 2002;359(9313):1221‐31. - PubMed
Hemmer 2006
-
- Hemmer B, Nessler S, Zhou D, Kieseier B, Hartung HP. Immunopathogenesis and immunotherapy of multiple sclerosis. Nature Clinical Practice Neurology 2006;2(4):201‐11. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kurtzke 1983
-
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33(11):1444‐52. - PubMed
Lefebvre 2009
-
- Lefebrvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Li 2005
-
- Li Z, Lim WK, Mahesh SP, Liu B, Nussenblatt RB. Cutting edge: in vivo blockade of human IL‐2 receptor induces expansion of CD56(bright) regulatory NK cells in patients with active uveitis. Journal of Immunology 2005;174(9):5187‐91. - PubMed
Martin 2012
-
- Martin R. Anti‐CD25 (daclizumab) monoclonal antibody therapy in relapsing‐remitting multiple sclerosis. Clinical Immunology 2012;142(1):9‐14. - PubMed
McDonald 2001
-
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of Neurology 2001;50(1):121‐7. - PubMed
Nelson 1998
-
- Nelson BH, Willerford DM. Biology of the interleukin‐2 receptor. Advances in Immunology 1998;70:1‐81. - PubMed
Polman 2005
-
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the 'McDonald' criteria. Annals of Neurology 2005;58(6):840‐6. - PubMed
Poser 1983
-
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13(3):227‐31. - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schippling 2008
-
- Schippling DS, Martin R. Spotlight on anti‐CD25: daclizumab in MS. International MS Journal 2008;15(3):94‐8. - PubMed
Webster 2010
Weinshenker 1989
-
- Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain 1989;112(1):133‐46. - PubMed
References to other published versions of this review
Liu 2010
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

