Conditioned media from adipose-tissue-derived mesenchymal stem cells downregulate degradative mediators induced by interleukin-1β in osteoarthritic chondrocytes
- PMID: 24363499
- PMCID: PMC3864089
- DOI: 10.1155/2013/357014
Conditioned media from adipose-tissue-derived mesenchymal stem cells downregulate degradative mediators induced by interleukin-1β in osteoarthritic chondrocytes
Abstract
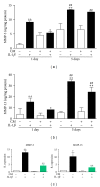
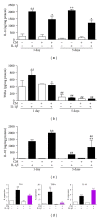
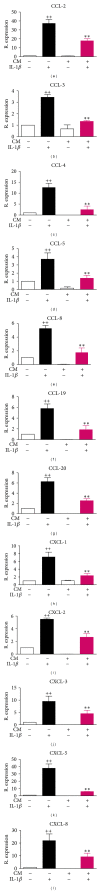
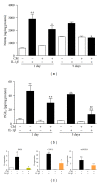
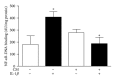
Osteoarthritis (OA) is the most frequent joint disorder and an important cause of disability. Recent studies have shown the potential of adipose-tissue-derived mesenchymal stem cells (AD-MSC) for cartilage repair. We have investigated whether conditioned medium from AD-MSC (CM) may regulate in OA chondrocytes a number of key mediators involved in cartilage degeneration. CM enhanced type II collagen expression in OA chondrocytes while decreasing matrix metalloproteinase (MMP) activity in cell supernatants as well as the levels of MMP-3 and MMP-13 proteins and mRNA in OA chondrocytes stimulated with interleukin- (IL-) 1β. In addition, CM increased IL-10 levels and counteracted the stimulating effects of IL-1β on the production of tumor necrosis factor-α, IL-6, prostaglandin E2, and NO measured as nitrite and the mRNA expression of these cytokines, CCL-2, CCL-3, CCL-4, CCL-5, CCL-8, CCL-19, CCL-20, CXCL-1, CXCL-2, CXCL-3, CXCL-5, CXCL-8, cyclooxygenase-2, microsomal prostaglandin E synthase-1, and inducible NO synthase. These effects may be dependent on the inhibition of nuclear factor-κB activation by CM. Our data demonstrate the chondroprotective actions of CM and provide support for further studies of this approach in joint disease.
Figures


References
-
- Goldring MB, Goldring SR. Osteoarthritis. Journal of Cellular Physiology. 2007;213(3):626–634. - PubMed
-
- Fernandes JC, Martel-Pelletier J, Pelletier J-P. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39(1-2):237–246. - PubMed
-
- Mengshol JA, Vincenti MP, Coon CI, et al. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis and Rheumatism. 2000;43:801–811. - PubMed
-
- Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis and Rheumatism. 2001;44:585–594. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

