Ophthalmologic complications of antiviral therapy in hepatitis C treatment
- PMID: 24363513
- PMCID: PMC3857445
- DOI: 10.3748/wjg.v19.i45.8227
Ophthalmologic complications of antiviral therapy in hepatitis C treatment
Abstract
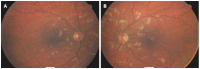
Antiviral therapy consisting of interferon-alpha and ribavirin for chronic hepatitis C infection is associated with multi-system side-effects. Ophthalmologic complications are common and can be classified into two groups: interferon-associated retinopathy and atypical adverse events. Interferon-associated retinopathy has been investigated by multiple observational studies that have found widely divergent results. The clinical importance of this complication is, consequently, controversial. This review examines the literature with the specific goal of identifying the most important ophthalmologic issues facing the hepatologist prescribing antiviral therapy. Accordingly, it assesses the incidence of interferon-associated retinopathy, as well as its risk factors, pathogenesis, clinical manifestations and options for management using data from the observational studies. The likely benefit of a screening program, especially one targeting patients with the highest risk of developing interferon-associated retinopathy, is analysed. Atypical ophthalmologic adverse events occur less frequently than interferon-associated retinopathy during antiviral therapy for chronic hepatitis C infection. They often, however, lead to irreversible vision loss. We examine the reports of these adverse events - in individual case reports or case series and in the observational studies investigating interferon-associated retinopathy - to describe the spectrum of these adverse events, the likely outcome for patients and to highlight the most important areas of future clinical research.
Keywords: Hepatitis C; Interferon; Ocular complications; Retinopathy.
© 2013 Baishideng Publishing Group Co., Limited. All rights reserved.
Figures
Similar articles
-
Interferon-α-induced retinopathy in chronic hepatitis C treatment: summary, considerations, and recommendations.Graefes Arch Clin Exp Ophthalmol. 2019 Mar;257(3):447-452. doi: 10.1007/s00417-018-04209-7. Epub 2018 Dec 13. Graefes Arch Clin Exp Ophthalmol. 2019. PMID: 30547319 Review.
-
Incidence and risk factors of retinopathy in Egyptian patients with chronic hepatitis C virus treated with pegylated interferon plus ribavirin.Int J Infect Dis. 2012 Jan;16(1):e67-71. doi: 10.1016/j.ijid.2011.09.022. Epub 2011 Nov 23. Int J Infect Dis. 2012. PMID: 22115957
-
Retinopathy in hepatitis C patients due to combination therapy with pegylated interferon and ribavirin.Jpn J Ophthalmol. 2009 Nov;53(6):598-602. doi: 10.1007/s10384-009-0738-8. Epub 2009 Dec 18. Jpn J Ophthalmol. 2009. PMID: 20020238
-
Retinal vascular complications associated with interferon-ribavirin therapy for chronic hepatitis C: A population-based study.Pharmacoepidemiol Drug Saf. 2018 Feb;27(2):191-198. doi: 10.1002/pds.4363. Epub 2017 Dec 6. Pharmacoepidemiol Drug Saf. 2018. PMID: 29210149
-
Visual side effects of pegylated interferon during therapy for chronic hepatitis C infection.J Clin Gastroenterol. 2004 Sep;38(8):717-22. doi: 10.1097/01.mcg.0000135897.30038.16. J Clin Gastroenterol. 2004. PMID: 15319658 Review.
Cited by
-
RELATIONSHIP BETWEEN UVEITIS, DIFFERENT TYPES OF VIRAL HEPATITIS, AND LIVER CIRRHOSIS: A 12-Year Nationwide Population-Based Cohort Study.Retina. 2016 Dec;36(12):2391-2398. doi: 10.1097/IAE.0000000000001103. Retina. 2016. PMID: 27870801 Free PMC article.
-
Interferon-α-induced retinopathy in chronic hepatitis C treatment: summary, considerations, and recommendations.Graefes Arch Clin Exp Ophthalmol. 2019 Mar;257(3):447-452. doi: 10.1007/s00417-018-04209-7. Epub 2018 Dec 13. Graefes Arch Clin Exp Ophthalmol. 2019. PMID: 30547319 Review.
-
Ocular Manifestations of COVID-19 (SARS-CoV-2): A Critical Review of Current Literature.In Vivo. 2020 Jun;34(3 Suppl):1619-1628. doi: 10.21873/invivo.11952. In Vivo. 2020. PMID: 32503820 Free PMC article. Review.
-
Nonarteritic anterior ischemic optic neuropathy associated with interferon and ribavirin in a patient with hepatitis C.Am J Ophthalmol Case Rep. 2016 Dec 5;5:52-55. doi: 10.1016/j.ajoc.2016.12.004. eCollection 2017 Apr. Am J Ophthalmol Case Rep. 2016. PMID: 29503948 Free PMC article.
-
Bilateral simultaneous anterior ischemic optic neuropathy, an extrahepatic manifestation of hepatitis C cured with direct acting antivirals.GMS Ophthalmol Cases. 2016 Apr 4;6:Doc05. doi: 10.3205/oc000042. eCollection 2016. GMS Ophthalmol Cases. 2016. PMID: 27625964 Free PMC article.
References
-
- Global surveillance and control of hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J Viral Hepat. 1999;6:35–47. - PubMed
-
- Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–115. - PubMed
-
- European Association of the Study of the Liver. 2011 European Association of the Study of the Liver hepatitis C virus clinical practice guidelines. Liver Int. 2012;32 Suppl 1:2–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical