Collective cell streams in epithelial monolayers depend on cell adhesion
- PMID: 24363603
- PMCID: PMC3866308
- DOI: 10.1088/1367-2630/15/7/075006
Collective cell streams in epithelial monolayers depend on cell adhesion
Abstract
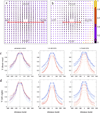
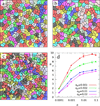
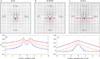
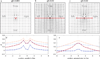
We report a spontaneously emerging, randomly oriented, collective streaming behavior within a monolayer culture of a human keratinocyte cell line, and explore the effect of modulating cell adhesions by perturbing the function of calcium-dependent cell adhesion molecules. We demonstrate that decreasing cell adhesion induces narrower and more anisotropic cell streams, reminiscent of decreasing the Taylor scale of turbulent liquids. To explain our empirical findings, we propose a cell-based model that represents the dual nature of cell-cell adhesions. Spring-like connections provide mechanical stability, while a cellular Potts model formalism represents surface-tension driven attachment. By changing the relevance and persistence of mechanical links between cells, we are able to explain the experimentally observed changes in emergent flow patterns.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
