Sperm proteomics: road to male fertility and contraception
- PMID: 24363670
- PMCID: PMC3864079
- DOI: 10.1155/2013/360986
Sperm proteomics: road to male fertility and contraception
Abstract
Spermatozoa are highly specialized cells that can be easily obtained and purified. Mature spermatozoa are transcriptionally and translationally inactive and incapable of protein synthesis. In addition, spermatozoa contain relatively higher amounts of membrane proteins compared to other cells; therefore, they are very suitable for proteomic studies. Recently, the application of proteomic approaches such as the two-dimensional polyacrylamide gel electrophoresis, mass spectrometry, and differential in-gel electrophoresis has identified several sperm-specific proteins. These findings have provided a further understanding of protein functions involved in different sperm processes as well as of the differentiation of normal state from an abnormal one. In addition, studies on the sperm proteome have demonstrated the importance of spermatozoal posttranslational modifications and their ability to induce physiological changes responsible for fertilization. Large-scale proteomic studies to identify hundreds to thousands of sperm proteins will ultimately result in the development of novel biomarkers that may help to detect fertility, the state of complete contraception, and beyond. Eventually, these protein biomarkers will allow for a better diagnosis of sperm dysfunctions and aid in drug development. This paper reviews the recent scientific publications available from the PubMed database to address sperm proteomics and its potential application to characterize male fertility and contraception.
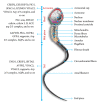
Figures


Similar articles
-
Proteomics: a subcellular look at spermatozoa.Reprod Biol Endocrinol. 2011 Mar 22;9:36. doi: 10.1186/1477-7827-9-36. Reprod Biol Endocrinol. 2011. PMID: 21426553 Free PMC article. Review.
-
Sperm proteome and reproductive technologies in mammals.Anim Reprod Sci. 2016 Oct;173:1-7. doi: 10.1016/j.anireprosci.2016.08.008. Epub 2016 Aug 23. Anim Reprod Sci. 2016. PMID: 27576173 Review.
-
The combined human sperm proteome: cellular pathways and implications for basic and clinical science.Hum Reprod Update. 2014 Jan-Feb;20(1):40-62. doi: 10.1093/humupd/dmt046. Epub 2013 Sep 29. Hum Reprod Update. 2014. PMID: 24082039 Review.
-
Sperm cell proteomics.Proteomics. 2009 Feb;9(4):1004-17. doi: 10.1002/pmic.200800588. Proteomics. 2009. PMID: 19212950 Review.
-
Proteomic Analyses of Human Sperm Cells: Understanding the Role of Proteins and Molecular Pathways Affecting Male Reproductive Health.Int J Mol Sci. 2020 Feb 27;21(5):1621. doi: 10.3390/ijms21051621. Int J Mol Sci. 2020. PMID: 32120839 Free PMC article. Review.
Cited by
-
A comprehensive proteomic approach to identifying capacitation related proteins in boar spermatozoa.BMC Genomics. 2014 Oct 14;15(1):897. doi: 10.1186/1471-2164-15-897. BMC Genomics. 2014. PMID: 25315394 Free PMC article.
-
Male caffeine and alcohol intake in relation to semen parameters and in vitro fertilization outcomes among fertility patients.Andrology. 2017 Mar;5(2):354-361. doi: 10.1111/andr.12310. Epub 2017 Feb 10. Andrology. 2017. PMID: 28187518 Free PMC article.
-
Characterization of the Testis-Specific Angiotensin Converting Enzyme (tACE)-Interactome during Bovine Sperm Capacitation.Curr Issues Mol Biol. 2022 Jan 17;44(1):449-469. doi: 10.3390/cimb44010031. Curr Issues Mol Biol. 2022. PMID: 35723410 Free PMC article.
-
Increased male fertility using fertility-related biomarkers.Sci Rep. 2015 Oct 22;5:15654. doi: 10.1038/srep15654. Sci Rep. 2015. PMID: 26489431 Free PMC article.
-
Intact Cell MALDI-TOF MS on Sperm: A Molecular Test For Male Fertility Diagnosis.Mol Cell Proteomics. 2016 Jun;15(6):1998-2010. doi: 10.1074/mcp.M116.058289. Epub 2016 Apr 4. Mol Cell Proteomics. 2016. PMID: 27044871 Free PMC article.
References
-
- Park YJ, Kwon WS, Oh SA, et al. Fertility-related proteomic profiling bull spermatozoa separated by percoll. Journal of Proteome Research. 2012;11(8):4162–4168. - PubMed
-
- Turek PJ. Practical approaches to the diagnosis and management of male infertility. Nature Clinical Practice Urology. 2005;2(5):226–238. - PubMed
-
- Sharlip ID, Jarow JP, Belker AM, et al. Best practice policies for male infertility. Fertility and Sterility. 2002;77(5):873–882. - PubMed
-
- Brewis IA. The potential importance of proteomics to research in reproduction. Human Reproduction. 1999;14(12):2927–2929. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

