Publication bias in recent meta-analyses
- PMID: 24363797
- PMCID: PMC3868709
- DOI: 10.1371/journal.pone.0081823
Publication bias in recent meta-analyses
Erratum in
- PLoS One. 2014;9(1). doi:10.1371/annotation/a65c0f61-eb99-42f0-828b-5a8662bce4f7
Abstract
Introduction: Positive results have a greater chance of being published and outcomes that are statistically significant have a greater chance of being fully reported. One consequence of research underreporting is that it may influence the sample of studies that is available for a meta-analysis. Smaller studies are often characterized by larger effects in published meta-analyses, which can be possibly explained by publication bias. We investigated the association between the statistical significance of the results and the probability of being included in recent meta-analyses.
Methods: For meta-analyses of clinical trials, we defined the relative risk as the ratio of the probability of including statistically significant results favoring the treatment to the probability of including other results. For meta-analyses of other studies, we defined the relative risk as the ratio of the probability of including biologically plausible statistically significant results to the probability of including other results. We applied a Bayesian selection model for meta-analyses that included at least 30 studies and were published in four major general medical journals (BMJ, JAMA, Lancet, and PLOS Medicine) between 2008 and 2012.
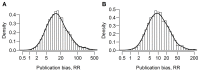
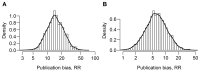
Results: We identified 49 meta-analyses. The estimate of the relative risk was greater than one in 42 meta-analyses, greater than two in 16 meta-analyses, greater than three in eight meta-analyses, and greater than five in four meta-analyses. In 10 out of 28 meta-analyses of clinical trials, there was strong evidence that statistically significant results favoring the treatment were more likely to be included. In 4 out of 19 meta-analyses of observational studies, there was strong evidence that plausible statistically significant outcomes had a higher probability of being included.
Conclusions: Publication bias was present in a substantial proportion of large meta-analyses that were recently published in four major medical journals.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

