Dissecting psychiatric spectrum disorders by generative embedding
- PMID: 24363992
- PMCID: PMC3863808
- DOI: 10.1016/j.nicl.2013.11.002
Dissecting psychiatric spectrum disorders by generative embedding
Abstract
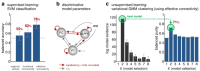
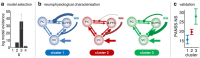
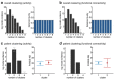
This proof-of-concept study examines the feasibility of defining subgroups in psychiatric spectrum disorders by generative embedding, using dynamical system models which infer neuronal circuit mechanisms from neuroimaging data. To this end, we re-analysed an fMRI dataset of 41 patients diagnosed with schizophrenia and 42 healthy controls performing a numerical n-back working-memory task. In our generative-embedding approach, we used parameter estimates from a dynamic causal model (DCM) of a visual-parietal-prefrontal network to define a model-based feature space for the subsequent application of supervised and unsupervised learning techniques. First, using a linear support vector machine for classification, we were able to predict individual diagnostic labels significantly more accurately (78%) from DCM-based effective connectivity estimates than from functional connectivity between (62%) or local activity within the same regions (55%). Second, an unsupervised approach based on variational Bayesian Gaussian mixture modelling provided evidence for two clusters which mapped onto patients and controls with nearly the same accuracy (71%) as the supervised approach. Finally, when restricting the analysis only to the patients, Gaussian mixture modelling suggested the existence of three patient subgroups, each of which was characterised by a different architecture of the visual-parietal-prefrontal working-memory network. Critically, even though this analysis did not have access to information about the patients' clinical symptoms, the three neurophysiologically defined subgroups mapped onto three clinically distinct subgroups, distinguished by significant differences in negative symptom severity, as assessed on the Positive and Negative Syndrome Scale (PANSS). In summary, this study provides a concrete example of how psychiatric spectrum diseases may be split into subgroups that are defined in terms of neurophysiological mechanisms specified by a generative model of network dynamics such as DCM. The results corroborate our previous findings in stroke patients that generative embedding, compared to analyses of more conventional measures such as functional connectivity or regional activity, can significantly enhance both the interpretability and performance of computational approaches to clinical classification.
Keywords: Balanced purity; Clinical validation; Clustering; Schizophrenia; Variational Bayes.
Figures




References
-
- Attias H. A variational Bayesian framework for graphical models. Adv. Neural Inf. Process. Syst. 2000;12(1–2):209–215.
-
- Barr M.S., Farzan F., Tran L.C., Chen R., Fitzgerald P.B., Daskalakis Z.J. Evidence for excessive frontal evoked gamma oscillatory activity in schizophrenia during working memory. Schizophr. Res. 2010;121(1–3):146–152. - PubMed
-
- Bishop C.M. Springer New York; 2007. Pattern Recognition and Machine Learning.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

