Neuroprotective function of 14-3-3 proteins in neurodegeneration
- PMID: 24364034
- PMCID: PMC3865737
- DOI: 10.1155/2013/564534
Neuroprotective function of 14-3-3 proteins in neurodegeneration
Abstract
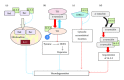
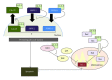
14-3-3 proteins are abundantly expressed adaptor proteins that interact with a vast number of binding partners to regulate their cellular localization and function. They regulate substrate function in a number of ways including protection from dephosphorylation, regulation of enzyme activity, formation of ternary complexes and sequestration. The diversity of 14-3-3 interacting partners thus enables 14-3-3 proteins to impact a wide variety of cellular and physiological processes. 14-3-3 proteins are broadly expressed in the brain, and clinical and experimental studies have implicated 14-3-3 proteins in neurodegenerative disease. A recurring theme is that 14-3-3 proteins play important roles in pathogenesis through regulating the subcellular localization of target proteins. Here, we review the evidence that 14-3-3 proteins regulate aspects of neurodegenerative disease with a focus on their protective roles against neurodegeneration.
Figures
Similar articles
-
14-3-3 Proteins Are on the Crossroads of Cancer, Aging, and Age-Related Neurodegenerative Disease.Int J Mol Sci. 2019 Jul 18;20(14):3518. doi: 10.3390/ijms20143518. Int J Mol Sci. 2019. PMID: 31323761 Free PMC article. Review.
-
Structure, Function, Involvement in Diseases and Targeting of 14-3-3 Proteins: An Update.Curr Med Chem. 2018;25(1):5-21. doi: 10.2174/0929867324666170426095015. Curr Med Chem. 2018. PMID: 28462702 Review.
-
Protein-protein interaction network with machine learning models and multiomics data reveal potential neurodegenerative disease-related proteins.Hum Mol Genet. 2020 May 28;29(8):1378-1387. doi: 10.1093/hmg/ddaa065. Hum Mol Genet. 2020. PMID: 32277755
-
14-3-3 proteins in neurodegeneration.Semin Cell Dev Biol. 2011 Sep;22(7):696-704. doi: 10.1016/j.semcdb.2011.08.005. Epub 2011 Sep 6. Semin Cell Dev Biol. 2011. PMID: 21920445 Review.
-
Maintaining the balance of TDP-43, mitochondria, and autophagy: a promising therapeutic strategy for neurodegenerative diseases.Transl Neurodegener. 2020 Oct 30;9(1):40. doi: 10.1186/s40035-020-00219-w. Transl Neurodegener. 2020. PMID: 33126923 Free PMC article. Review.
Cited by
-
Proteomic approach for understanding milder neurotoxicity of Carfilzomib against Bortezomib.Sci Rep. 2018 Nov 5;8(1):16318. doi: 10.1038/s41598-018-34507-3. Sci Rep. 2018. PMID: 30397214 Free PMC article.
-
Comprehensive Analysis of Proteasomal Complexes in Mouse Brain Regions Detects ENO2 as a Potential Partner of the Proteasome in the Striatum.Cell Mol Neurobiol. 2022 Oct;42(7):2305-2319. doi: 10.1007/s10571-021-01106-2. Epub 2021 May 26. Cell Mol Neurobiol. 2022. PMID: 34037901 Free PMC article.
-
Formation of amyloid fibrils by the regulatory 14-3-3ζ protein.Open Biol. 2024 Jan;14(1):230285. doi: 10.1098/rsob.230285. Epub 2024 Jan 17. Open Biol. 2024. PMID: 38228169 Free PMC article.
-
Differential mitochondrial protein interaction profile between human translocator protein and its A147T polymorphism variant.PLoS One. 2022 May 6;17(5):e0254296. doi: 10.1371/journal.pone.0254296. eCollection 2022. PLoS One. 2022. PMID: 35522669 Free PMC article.
-
Microdissected Pyramidal Cell Proteomics of Alzheimer Brain Reveals Alterations in Creatine Kinase B-Type, 14-3-3-γ, and Heat Shock Cognate 71.Front Aging Neurosci. 2021 Nov 19;13:735334. doi: 10.3389/fnagi.2021.735334. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34867272 Free PMC article.
References
-
- Moore BW, Perez VJ. Specific acidic proteins of the nervous system. In: Carlson FD, editor. Physiological and Biochemical Aspects of Nervous Integration. 1968. pp. 343–360.
-
- Boston PF, Jackson P, Thompson RJ. Human 14-3-3 protein: radioimmunoassay, tissue distribution, and cerebrospinal fluid levels in patients with neurological disorders. Journal of Neurochemistry. 1982;38(5):1475–1482. - PubMed
-
- Berg D, Holzmann C, Riess O. 14-3-3 proteins in the nervous system. Nature Reviews Neuroscience. 2003;4(9):752–762. - PubMed
-
- Martin H, Patel Y, Jones D, Howell S, Robinson K, Aitken A. Antibodies against the major brain isoforms of 14-3-3 protein. An antibody specific for the N-acetylated amino-terminus of a protein. FEBS Letters. 1993;331(3):296–303. - PubMed
-
- Jones DH, Ley S, Aitken A. Isoforms of 14-3-3 protein can form homo- and heterodimers in vivo and in vitro: implications for function as adapter proteins. FEBS Letters. 1995;368(1):55–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

