Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial
- PMID: 24366103
- PMCID: PMC3940333
- DOI: 10.1001/jamaneurol.2013.5528
Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial
Abstract
Importance: Convergent biological, epidemiological, and clinical data identified urate elevation as a candidate strategy for slowing disability progression in Parkinson disease (PD).
Objective: To determine the safety, tolerability, and urate-elevating capability of the urate precursor inosine in early PD and to assess its suitability and potential design features for a disease-modification trial.
Design, setting, and participants: The Safety of Urate Elevation in PD (SURE-PD) study, a randomized, double-blind, placebo-controlled, dose-ranging trial of inosine, enrolled participants from 2009 to 2011 and followed them for up to 25 months at outpatient visits to 17 credentialed clinical study sites of the Parkinson Study Group across the United States. Seventy-five consenting adults (mean age, 62 years; 55% women) with early PD not yet requiring symptomatic treatment and a serum urate concentration less than 6 mg/dL (the approximate population median) were enrolled.
Interventions: Participants were randomized to 1 of 3 treatment arms: placebo or inosine titrated to produce mild (6.1-7.0 mg/dL) or moderate (7.1-8.0 mg/dL) serum urate elevation using 500-mg capsules taken orally up to 2 capsules 3 times per day. They were followed for up to 24 months (median, 18 months) while receiving the study drug plus 1 washout month.
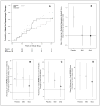
Main outcomes and measures: The prespecified primary outcomes were absence of unacceptable serious adverse events (safety), continued treatment without adverse event requiring dose reduction (tolerability), and elevation of urate assessed serially in serum and once (at 3 months) in cerebrospinal fluid. RESULTS Serious adverse events (17), including infrequent cardiovascular events, occurred at the same or lower rates in the inosine groups relative to placebo. No participant developed gout and 3 receiving inosine developed symptomatic urolithiasis. Treatment was tolerated by 95% of participants at 6 months, and no participant withdrew because of an adverse event. Serum urate rose by 2.3 and 3.0 mg/dL in the 2 inosine groups (P < .001 for each) vs placebo, and cerebrospinal fluid urate level was greater in both inosine groups (P = .006 and <.001, respectively). Secondary analyses demonstrated nonfutility of inosine treatment for slowing disability.
Conclusions and relevance: Inosine was generally safe, tolerable, and effective in raising serum and cerebrospinal fluid urate levels in early PD. The findings support advancing to more definitive development of inosine as a potential disease-modifying therapy for PD.
Trial registration: clinicaltrials.gov Identifier: NCT00833690.
Conflict of interest statement
Figures

References
-
- Gong L, Zhang QL, Zhang N, et al. Neuroprotection by urate on 6-OHDA-lesioned rat model of Parkinson’s disease: linking to Akt/GSK3β signaling pathway. J Neurochem. 2012 Dec;123(5):876–85. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

