How to build a grid cell
- PMID: 24366132
- PMCID: PMC3866442
- DOI: 10.1098/rstb.2012.0520
How to build a grid cell
Abstract
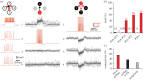
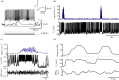
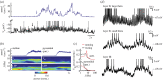
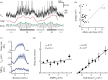
Neurons in the medial entorhinal cortex fire action potentials at regular spatial intervals, creating a striking grid-like pattern of spike rates spanning the whole environment of a navigating animal. This remarkable spatial code may represent a neural map for path integration. Recent advances using patch-clamp recordings from entorhinal cortex neurons in vitro and in vivo have revealed how the microcircuitry in the medial entorhinal cortex may contribute to grid cell firing patterns, and how grid cells may transform synaptic inputs into spike output during firing field crossings. These new findings provide key insights into the ingredients necessary to build a grid cell.
Keywords: entorhinal cortex; grid cell; neural circuit; patch clamp; path integration; spatial navigation.
Figures

Similar articles
-
Cellular mechanisms of spatial navigation in the medial entorhinal cortex.Nat Neurosci. 2013 Mar;16(3):325-31. doi: 10.1038/nn.3340. Epub 2013 Feb 10. Nat Neurosci. 2013. PMID: 23396102
-
Interspike Intervals Reveal Functionally Distinct Cell Populations in the Medial Entorhinal Cortex.J Neurosci. 2015 Aug 5;35(31):10963-76. doi: 10.1523/JNEUROSCI.0276-15.2015. J Neurosci. 2015. PMID: 26245960 Free PMC article.
-
Recurrent inhibitory circuitry as a mechanism for grid formation.Nat Neurosci. 2013 Mar;16(3):318-24. doi: 10.1038/nn.3310. Epub 2013 Jan 20. Nat Neurosci. 2013. PMID: 23334580
-
Independence of landmark and self-motion-guided navigation: a different role for grid cells.Philos Trans R Soc Lond B Biol Sci. 2013 Dec 23;369(1635):20130370. doi: 10.1098/rstb.2013.0370. Print 2014 Feb 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24366147 Free PMC article. Review.
-
Ten Years of Grid Cells.Annu Rev Neurosci. 2016 Jul 8;39:19-40. doi: 10.1146/annurev-neuro-070815-013824. Epub 2016 Mar 9. Annu Rev Neurosci. 2016. PMID: 27023731 Review.
Cited by
-
An Analysis of Waves Underlying Grid Cell Firing in the Medial Enthorinal Cortex.J Math Neurosci. 2017 Aug 25;7(1):9. doi: 10.1186/s13408-017-0051-7. J Math Neurosci. 2017. PMID: 28842863 Free PMC article.
-
Multi-Scale Extension in an Entorhinal-Hippocampal Model for Cognitive Map Building.Front Neurorobot. 2021 Jan 14;14:592057. doi: 10.3389/fnbot.2020.592057. eCollection 2020. Front Neurorobot. 2021. PMID: 33519410 Free PMC article.
-
Space in the brain: how the hippocampal formation supports spatial cognition.Philos Trans R Soc Lond B Biol Sci. 2013 Dec 23;369(1635):20120510. doi: 10.1098/rstb.2012.0510. Print 2014 Feb 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24366125 Free PMC article. Review.
-
Active dendritic integration as a mechanism for robust and precise grid cell firing.Nat Neurosci. 2017 Aug;20(8):1114-1121. doi: 10.1038/nn.4582. Epub 2017 Jun 19. Nat Neurosci. 2017. PMID: 28628104 Free PMC article.
-
Simulation of oscillatory dynamics induced by an approximation of grid cell output.Rev Neurosci. 2022 Nov 4;34(5):517-532. doi: 10.1515/revneuro-2022-0107. Print 2023 Jul 26. Rev Neurosci. 2022. PMID: 36326795 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

