Neuronal rebound spiking, resonance frequency and theta cycle skipping may contribute to grid cell firing in medial entorhinal cortex
- PMID: 24366135
- PMCID: PMC3866445
- DOI: 10.1098/rstb.2012.0523
Neuronal rebound spiking, resonance frequency and theta cycle skipping may contribute to grid cell firing in medial entorhinal cortex
Abstract
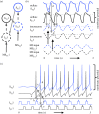
Data show a relationship of cellular resonance and network oscillations in the entorhinal cortex to the spatial periodicity of grid cells. This paper presents a model that simulates the resonance and rebound spiking properties of entorhinal neurons to generate spatial periodicity dependent upon phasic input from medial septum. The model shows that a difference in spatial periodicity can result from a difference in neuronal resonance frequency that replicates data from several experiments. The model also demonstrates a functional role for the phenomenon of theta cycle skipping in the medial entorhinal cortex.
Keywords: entorhinal cortex; grid cells; head direction; place cells; rat; theta rhythm.
Figures



References
-
- Burgess N, Barry C, Jeffery KJ, O'Keefe J. 2005. A grid and place cell model of path integration utilizing phase precession versus theta. Poster presented at the Computational Cognitive Neuroscience Conference, Washington DC, 10–11 November 2005. See http://cdn.f1000.com/posters/docs/225.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
