The Nod1, Nod2, and Rip2 axis contributes to host immune defense against intracellular Acinetobacter baumannii infection
- PMID: 24366254
- PMCID: PMC3958010
- DOI: 10.1128/IAI.01459-13
The Nod1, Nod2, and Rip2 axis contributes to host immune defense against intracellular Acinetobacter baumannii infection
Abstract
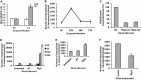
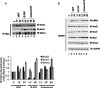
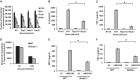
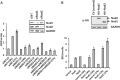
Acinetobacter baumannii is a major extensively drug-resistant lethal human nosocomial bacterium. However, the host innate immune mechanisms controlling A. baumannii are not well understood. Although viewed as an extracellular pathogen, A. baumannii can also invade and survive intracellularly. However, whether host innate immune pathways sensing intracellular bacteria contribute to immunity against A. baumannii is not known. Here, we provide evidence for the first time that intracellular antibacterial innate immune receptors Nod1 and Nod2, and their adaptor Rip2, play critical roles in the sensing and clearance of A. baumannii by human airway epithelial cells in vitro. A. baumannii infection upregulated Rip2 expression. Silencing of Nod1, Nod2, and Rip2 expression profoundly increased intracellular invasion and prolonged the multiplication and survival of A. baumannii in lung epithelial cells. Notably, the Nod1/2-Rip2 axis did not contribute to the control of A. baumannii infection of human macrophages, indicating that they play cell type-specific roles. The Nod1/2-Rip2 axis was needed for A. baumannii infection-induced activation of NF-κB but not mitogen-activated protein kinases. Moreover, the Nod1/2-Rip2 axis was critical to induce optimal cytokine and chemokine responses to A. baumannii infection. Mechanistic studies showed that the Nod1/2 pathway contributed to the innate control of A. baumannii infection through the production of β-defensin 2 by airway epithelial cells. This study revealed new insights into the immune control of A. baumannii and may contribute to the development of effective immune therapeutics and vaccines against A. baumannii.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

