Huntingtin-associated protein 1 regulates exocytosis, vesicle docking, readily releasable pool size and fusion pore stability in mouse chromaffin cells
- PMID: 24366265
- PMCID: PMC3979608
- DOI: 10.1113/jphysiol.2013.268342
Huntingtin-associated protein 1 regulates exocytosis, vesicle docking, readily releasable pool size and fusion pore stability in mouse chromaffin cells
Abstract
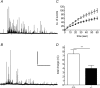
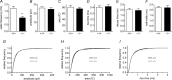
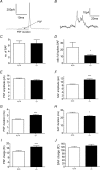
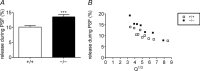
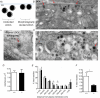
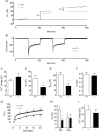
Huntingtin-associated protein 1 (HAP1) was initially established as a neuronal binding partner of huntingtin, mutations in which underlie Huntington's disease. Subcellular localization and protein interaction data indicate that HAP1 may be important in vesicle trafficking and cell signalling. In this study, we establish that HAP1 is important in several steps of exocytosis in adrenal chromaffin cells. Using carbon-fibre amperometry, we measured single vesicle exocytosis in chromaffin cells obtained from HAP1(-/-) and HAP1(+/+) littermate mice. Numbers of Ca(2+)-dependent and Ca(2+)-independent full fusion events in HAP1(-/-) cells are significantly decreased compared with those in HAP1(+/+) cells. We observed no change in the frequency of 'kiss-and-run' fusion events or in Ca(2+) entry. Whereas release per full fusion event is unchanged in HAP1(-/-) cells, early fusion pore duration is prolonged, as indicated by the increased duration of pre-spike foot signals. Kiss-and-run events have a shorter duration, indicating opposing roles for HAP1 in the stabilization of the fusion pore during full fusion and transient fusion, respectively. We use electron microscopy to demonstrate a reduction in the number of vesicles docked at the plasma membrane of HAP1(-/-) cells, where membrane capacitance measurements reveal the readily releasable pool of vesicles to be reduced in size. Our study therefore illustrates that HAP1 regulates exocytosis by influencing the morphological docking of vesicles at the plasma membrane, the ability of vesicles to be released rapidly upon stimulation, and the early stages of fusion pore formation.
Figures


References
-
- Aminoff MJ, Trenchard A, Turner P, Wood WG, Hills M. Plasma uptake of dopamine and 5-hydroxytryptamine and plasma-catecholamine levels in patients with Huntington's chorea. Lancet. 1974;2:1115–1116. - PubMed
-
- Bertaux F, Sharp AH, Ross CA, Lehrach H, Bates GP, Wanker E. HAP1–huntingtin interactions do not contribute to the molecular pathology in Huntington's disease transgenic mice. FEBS Letters. 1998;426:229–232. - PubMed
-
- Bjorkqvist M, Fex M, Renstrom E, Wierup N, Petersen A, Gil J, Bacos K, Popovic N, Li JY, Sundler F, Brundin P, Mulder H. The R6/2 transgenic mouse model of Huntington's disease develops diabetes due to deficient β-cell mass and exocytosis. Hum Mol Genet. 2005;14:565–574. - PubMed
-
- Block-Galarza J, Chase KO, Sapp E, Vaughn KT, Vallee RB, DiFiglia M, Aronin N. Fast transport and retrograde movement of huntingtin and HAP 1 in axons. Neuroreport. 1997;8:2247–2251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

