Pazopanib exposure decreases as a result of an ifosfamide-dependent drug-drug interaction: results of a phase I study
- PMID: 24366297
- PMCID: PMC3929878
- DOI: 10.1038/bjc.2013.798
Pazopanib exposure decreases as a result of an ifosfamide-dependent drug-drug interaction: results of a phase I study
Abstract
Background: The vascular endothelial growth factor receptor (VEGFR) pathway plays a pivotal role in solid malignancies and is probably involved in chemotherapy resistance. Pazopanib, inhibitor of, among other receptors, VEGFR1-3, has activity as single agent and is attractive to enhance anti-tumour activity of chemotherapy. We conducted a dose-finding and pharmacokinetic (PK)/pharmacodynamics study of pazopanib combined with two different schedules of ifosfamide.
Methods: In a 3+3+3 design, patients with advanced solid tumours received escalating doses of oral pazopanib combined with ifosfamide either given 3 days continuously or given 3-h bolus infusion daily for 3 days (9 g m(-2) per cycle, every 3 weeks). Pharmacokinetic data of ifosfamide and pazopanib were obtained. Plasma levels of placental-derived growth factor (PlGF), vascular endothelial growth factor-A (VEGF-A), soluble VEGFR2 (sVEGFR2) and circulating endothelial cells were monitored as biomarkers.
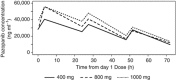
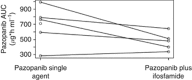
Results: Sixty-one patients were included. Pazopanib with continuous ifosfamide infusion appeared to be safe up to 1000 mg per day, while combination with bolus infusion ifosfamide turned out to be too toxic based on a variety of adverse events. Ifosfamide-dependent decline in pazopanib exposure was observed. Increases in PlGF and VEGF-A with concurrent decline in sVEGFR2 levels, consistent with pazopanib-mediated VEGFR2 inhibition, were observed after addition of ifosfamide.
Conclusion: Continuous as opposed to bolus infusion ifosfamide can safely be combined with pazopanib. Ifosfamide co-administration results in lower exposure to pazopanib, not hindering biological effects of pazopanib. Recommended dose of pazopanib for further studies combined with 3 days continuous ifosfamide (9 g m(-2) per cycle, every 3 weeks) is 800 mg daily.
Figures


Similar articles
-
Cytokine and angiogenic factors associated with efficacy and toxicity of pazopanib in advanced soft-tissue sarcoma: an EORTC-STBSG study.Br J Cancer. 2012 Aug 7;107(4):639-45. doi: 10.1038/bjc.2012.328. Epub 2012 Jul 17. Br J Cancer. 2012. PMID: 22805326 Free PMC article. Clinical Trial.
-
Combination therapy with pazopanib and tivantinib modulates VEGF and c-MET levels in refractory advanced solid tumors.Invest New Drugs. 2021 Dec;39(6):1577-1586. doi: 10.1007/s10637-021-01138-x. Epub 2021 Jun 28. Invest New Drugs. 2021. PMID: 34180036 Free PMC article. Clinical Trial.
-
Phase 1 study of pazopanib alone or combined with lapatinib in Japanese patients with solid tumors.Cancer Chemother Pharmacol. 2014 Apr;73(4):673-83. doi: 10.1007/s00280-014-2374-3. Epub 2014 Jan 24. Cancer Chemother Pharmacol. 2014. PMID: 24464355 Clinical Trial.
-
(Pre-)clinical pharmacology and activity of pazopanib, a novel multikinase angiogenesis inhibitor.Oncologist. 2010;15(6):539-47. doi: 10.1634/theoncologist.2009-0274. Epub 2010 May 28. Oncologist. 2010. PMID: 20511320 Free PMC article. Review.
-
Pazopanib for the treatment of metastatic renal cell carcinoma.Clin Ther. 2012 Mar;34(3):511-20. doi: 10.1016/j.clinthera.2012.01.014. Epub 2012 Feb 16. Clin Ther. 2012. PMID: 22341567 Review.
Cited by
-
Clinical Pharmacokinetics and Pharmacodynamics of Pazopanib: Towards Optimized Dosing.Clin Pharmacokinet. 2017 Sep;56(9):987-997. doi: 10.1007/s40262-017-0510-z. Clin Pharmacokinet. 2017. PMID: 28185218 Free PMC article. Review.
-
Impact of CYP3A4*22 on Pazopanib Pharmacokinetics in Cancer Patients.Clin Pharmacokinet. 2019 May;58(5):651-658. doi: 10.1007/s40262-018-0719-5. Clin Pharmacokinet. 2019. PMID: 30367352 Free PMC article. Clinical Trial.
-
Recent advances on anti-angiogenic multi-receptor tyrosine kinase inhibitors in osteosarcoma and Ewing sarcoma.Front Oncol. 2023 Mar 13;13:1013359. doi: 10.3389/fonc.2023.1013359. eCollection 2023. Front Oncol. 2023. PMID: 36994209 Free PMC article. Review.
-
(Lymph)angiogenic influences on hematopoietic cells in acute myeloid leukemia.Exp Mol Med. 2014 Nov 21;46(11):e122. doi: 10.1038/emm.2014.72. Exp Mol Med. 2014. PMID: 25412683 Free PMC article. Review.
-
A Phase I, Dose-Escalation Trial of Pazopanib in Combination with Cisplatin in Patients with Advanced Solid Tumors: A UNICANCER Study.Oncol Ther. 2016;4(2):211-223. doi: 10.1007/s40487-016-0027-x. Epub 2016 Aug 18. Oncol Ther. 2016. PMID: 28261651 Free PMC article.
References
-
- Boddy AV, Cole M, Pearson AD, Idle JR. The kinetics of the auto-induction of ifosfamide metabolism during continuous infusion. Cancer Chemother Pharmacol. 1995;36:53–60. - PubMed
-
- Boucher Y, Jain RK. Microvascular pressure is the principal driving force for interstitial hypertension in solid tumors: implications for vascular collapse. Cancer Res. 1992;52:5110–5114. - PubMed
-
- Burris HA, 3rd, Dowlati A, Moss RA, Infante JR, Jones SF, Spigel DR, Levinson KT, Lindquist D, Gainer SD, Dar MM, Suttle AB, Ball HA, Tan AR. Phase I study of pazopanib in combination with paclitaxel and carboplatin given every 21 days in patients with advanced solid tumors. Mol Cancer Ther. 2012;11:1820–1828. - PubMed
-
- Du Bois A, Vergote I, Wimberger P, Ray-Coquard I, Harter P, Curtis LB, Mitrica I. Open-label feasibility study of pazopanib, carboplatin, and paclitaxel in women with newly diagnosed, untreated, gynaecologic tumours: a phase I/II trial of the AGO study group. Br J Cancer. 2012;106:629–632. - PMC - PubMed
-
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

