USP3 inhibits type I interferon signaling by deubiquitinating RIG-I-like receptors
- PMID: 24366338
- PMCID: PMC3975496
- DOI: 10.1038/cr.2013.170
USP3 inhibits type I interferon signaling by deubiquitinating RIG-I-like receptors
Abstract
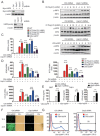
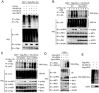
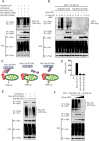
Lysine 63 (K63)-linked ubiquitination of RIG-I plays a critical role in the activation of type I interferon pathway, yet the molecular mechanism responsible for its deubiquitination is still poorly understood. Here we report that the deubiquitination enzyme ubiquitin-specific protease 3 (USP3) negatively regulates the activation of type I interferon signaling by targeting RIG-I. Knockdown of USP3 specifically enhanced K63-linked ubiquitination of RIG-I, upregulated the phosphorylation of IRF3 and augmented the production of type I interferon cytokines and antiviral immunity. We further show that there is no interaction between USP3 and RIG-I-like receptors (RLRs) in unstimulated or uninfected cells, but upon viral infection or ligand stimulation, USP3 binds to the caspase activation recruitment domain of RLRs and then cleaves polyubiquitin chains through cooperation of its zinc-finger Ub-binding domain and USP catalytic domains. Mutation analysis reveals that binding of USP3 to polyubiquitin chains on RIG-I is a prerequisite step for its cleavage of polyubiquitin chains. Our findings identify a previously unrecognized role of USP3 in RIG-I activation and provide insights into the mechanisms by which USP3 inhibits RIG-I signaling and antiviral immunity.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

