Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death
- PMID: 24366341
- PMCID: PMC3879712
- DOI: 10.1038/cr.2013.171
Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death
Abstract
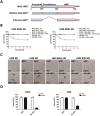
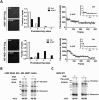
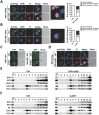
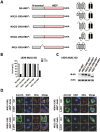
Mixed lineage kinase domain-like protein (MLKL) was identified to function downstream of receptor interacting protein 3 (RIP3) in tumor necrosis factor-α (TNF)-induced necrosis (also called necroptosis). However, how MLKL functions to mediate necroptosis is unknown. By reconstitution of MLKL function in MLKL-knockout cells, we showed that the N-terminus of MLKL is required for its function in necroptosis. The oligomerization of MLKL in TNF-treated cells is essential for necroptosis, as artificially forcing MLKL together by using the hormone-binding domain (HBD*) triggers necroptosis. Notably, forcing together the N-terminal domain (ND) but not the C-terminal kinase domain of MLKL causes necroptosis. Further deletion analysis showed that the four-α-helix bundle of MLKL (1-130 amino acids) is sufficient to trigger necroptosis. Both the HBD*-mediated and TNF-induced complexes of MLKL(ND) or MLKL are tetramers, and translocation of these complexes to lipid rafts of the plasma membrane precedes cell death. The homo-oligomerization is required for MLKL translocation and the signal sequence for plasma membrane location is located in the junction of the first and second α-helices of MLKL. The plasma membrane translocation of MLKL or MLKL(ND) leads to sodium influx, and depletion of sodium from the cell culture medium inhibits necroptosis. All of the above phenomena were not seen in apoptosis. Thus, the MLKL oligomerization leads to translocation of MLKL to lipid rafts of plasma membrane, and the plasma membrane MLKL complex acts either by itself or via other proteins to increase the sodium influx, which increases osmotic pressure, eventually leading to membrane rupture.
Figures


References
-
- Laster SM, Wood JG, Gooding LR. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988;141:2629–2634. - PubMed
-
- Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. - PubMed
-
- Degterev A, Huang Z, Boyce M, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. - PubMed
-
- Haas TL, Emmerich CH, Gerlach B, et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol Cell. 2009;36:831–844. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

