Thrombosis: tangled up in NETs
- PMID: 24366358
- PMCID: PMC4007606
- DOI: 10.1182/blood-2013-10-463646
Thrombosis: tangled up in NETs
Abstract
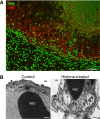
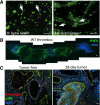
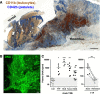
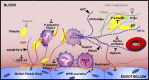
The contributions by blood cells to pathological venous thrombosis were only recently appreciated. Both platelets and neutrophils are now recognized as crucial for thrombus initiation and progression. Here we review the most recent findings regarding the role of neutrophil extracellular traps (NETs) in thrombosis. We describe the biological process of NET formation (NETosis) and how the extracellular release of DNA and protein components of NETs, such as histones and serine proteases, contributes to coagulation and platelet aggregation. Animal models have unveiled conditions in which NETs form and their relation to thrombogenesis. Genetically engineered mice enable further elucidation of the pathways contributing to NETosis at the molecular level. Peptidylarginine deiminase 4, an enzyme that mediates chromatin decondensation, was identified to regulate both NETosis and pathological thrombosis. A growing body of evidence reveals that NETs also form in human thrombosis and that NET biomarkers in plasma reflect disease activity. The cell biology of NETosis is still being actively characterized and may provide novel insights for the design of specific inhibitory therapeutics. After a review of the relevant literature, we propose new ways to approach thrombolysis and suggest potential prophylactic and therapeutic agents for thrombosis.
Figures

References
-
- Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. - PubMed
-
- Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8(4):668–676. - PubMed
-
- Saitoh T, Komano J, Saitoh Y, et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12(1):109–116. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

