JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes
- PMID: 24366362
- PMCID: PMC3945864
- DOI: 10.1182/blood-2013-11-539098
JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes
Abstract
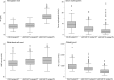
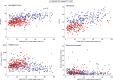
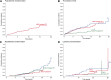
Patients with essential thrombocythemia may carry JAK2 (V617F), an MPL substitution, or a calreticulin gene (CALR) mutation. We studied biologic and clinical features of essential thrombocythemia according to JAK2 or CALR mutation status and in relation to those of polycythemia vera. The mutant allele burden was lower in JAK2-mutated than in CALR-mutated essential thrombocythemia. Patients with JAK2 (V617F) were older, had a higher hemoglobin level and white blood cell count, and lower platelet count and serum erythropoietin than those with CALR mutation. Hematologic parameters of patients with JAK2-mutated essential thrombocythemia or polycythemia vera were related to the mutant allele burden. While no polycythemic transformation was observed in CALR-mutated patients, the cumulative risk was 29% at 15 years in those with JAK2-mutated essential thrombocythemia. There was no significant difference in myelofibrotic transformation between the 2 subtypes of essential thrombocythemia. Patients with JAK2-mutated essential thrombocythemia and those with polycythemia vera had a similar risk of thrombosis, which was twice that of patients with the CALR mutation. These observations are consistent with the notion that JAK2-mutated essential thrombocythemia and polycythemia vera represent different phenotypes of a single myeloproliferative neoplasm, whereas CALR-mutated essential thrombocythemia is a distinct disease entity.
Figures

Comment in
-
Two faces of ET: CALR and JAK2.Blood. 2014 Mar 6;123(10):1438-40. doi: 10.1182/blood-2014-01-547596. Blood. 2014. PMID: 24627549
References
-
- Swerdlow SH, Campo E, Harris NL, et al. Lyon: IARC; 2008. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues.
-
- James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. - PubMed
-
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. - PubMed
-
- Cross NC. Genetic and epigenetic complexity in myeloproliferative neoplasms. Hematology Am Soc Hematol Educ Program. 2011;2011:208–214. - PubMed
-
- Passamonti F, Elena C, Schnittger S, et al. Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood. 2011;117(10):2813–2816. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

