A critical role for CK2 in cytokine-induced activation of NFκB in pancreatic β cell death
- PMID: 24366643
- PMCID: PMC4145192
- DOI: 10.1007/s12020-013-0133-6
A critical role for CK2 in cytokine-induced activation of NFκB in pancreatic β cell death
Abstract
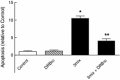
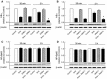
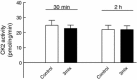
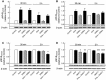
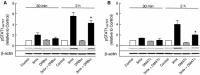
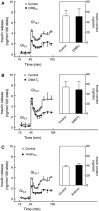
This study aimed to assess the role of constitutive protein kinase CK2 in cytokine-induced activation of NFκB in pancreatic β cell death. The CK2 inhibitors DRB (5,6-dichloro-1-β-D-ribofuranosylbenzimidazole) (50 μM) and DMAT (2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole) (5 μM), which decreased CK2 activity by approx. 65 %, rescued INS-1E β cells and mouse islets from cytokine (IL-1β, TNF-α plus IFN-γ)-induced β cell death without affecting H2O2- or palmitate-induced β cell death. Western blot analysis revealed that while DRB or DMAT did not influence cytokine-induced IκBα degradation, they inhibited NFκB-dependent IκBα resynthesis, demonstrating that cytokine-induced NFκB activity is dependent on CK2. Both DRB and DMAT inhibited the constitutive phosphorylation of NFκB p65 at serine 529, while leaving cytokine-induced phosphorylations of NFκB p65 at serines 276 and 536 unaltered. In comparison, putative phosphorylation sites for CK2 on HDACs 1, 2, and 3 at serines 421/423, 394, and 424, respectively, which may stimulate NFκB transcriptional activity, were unchanged by cytokines and CK2 inhibitors. Whereas IL-1β and TNF-α stimulate IκBα degradation and NFκB activation, IFN-γ potentiates cytokine-induced β cell death through activation of STAT1. DRB and DMAT inhibited IFN-γ-stimulated phosphorylation of STAT1 at serine 727, while leaving IFN-γ-induced phosphorylation of STAT1 at tyrosine 701 unaffected. Inhibition of cytokine-induced β cell death by CK2 inhibitors was, however, not dependent on IFN-γ, and IFN-γ did not affect CK2-dependent IκBα turnover. In conclusion, it is suggested that cytokine-induced activation of NFκB in β cells is dependent on CK2 activity, which phosphorylates NFκB p65 at serine 529.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

