Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START)
- PMID: 24366758
- PMCID: PMC3895196
- DOI: 10.1007/s00432-013-1563-5
Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START)
Abstract
Purpose: Cisplatin plus 5-fluorouracil has been globally accepted as a standard regimen for the treatment for advanced gastric cancer. However, cisplatin has several disadvantages, including renal toxicity and the need for admission. S-1 plus cisplatin has become a standard treatment for advanced gastric cancer in East Asia. This phase III study was designed to evaluate the potential benefits of adding docetaxel to S-1 without a platinum compound in patients with advanced gastric cancer.
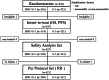
Methods: Patients were randomly assigned to receive docetaxel plus S-1 or S-1 alone. The docetaxel plus S-1 group received docetaxel on day 1 and oral S-1 on days 1-14 of a 21-day cycle. The S-1 alone group received oral S-1 on days 1-28 of a 42-day cycle. The primary end point was overall survival.
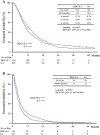
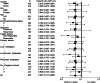
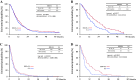
Results: Of the 639 patients enrolled, 635 were eligible for analysis. The median overall survival was 12.5 months in the docetaxel plus S-1 group and 10.8 months in the S-1 alone group (p = 0.032). The median progression-free survival was 5.3 months in the docetaxel plus S-1 group and 4.2 months in the S-1 alone group (p = 0.001). As for adverse events, neutropenia was more frequent in the docetaxel plus S-1 group, but remained manageable.
Conclusion: As first-line treatment for advanced gastric cancer, docetaxel plus S-1 significantly improves median overall and progression-free survival as compared with S-1 alone. (ClinicalTrials.gov number: NCT00287768).
Conflict of interest statement
Yeul Hong Kim has received the grants from Sanofi, Jeil Pharmaceutical, Pfizer, Astra Zeneca, Merck Serono, Novartis, and Roche, as well as honoraria for lectures from Sanofi, Jeil Pharmaceutical, Merck Serono, and Roche and a travel grant from Sanofi. Masashi Fujii has received consulting fees and honoraria for lectures from Taiho Pharmaceutical. Hyun Cheol Chung has received a grant from Sanofi. Akinori Takagane has received travel and hotel grants from JACCRO. Kazuhiro Yoshida has received grants from Chugai Pharmaceutical, Kyowa Hakko Kirin, Pfizer, Sanofi, Taiho Pharmaceutical, and Yakult Honsha, as well as honoraria for lectures from Chugai Pharmaceutical, Kyowa Hakko Kirin, Novartis, Pfizer, Sanofi, Taiho Pharmaceutical, and Yakult Honsha. He also has consultant or advisory relationships with Taiho Pharmaceutical and Roche. All other authors have declared no conflicts of interest.
Figures
References
-
- Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, Vynnychenko I, Garin A, Lang I, Falcon S (2010) Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol 28:1547–1553 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

