Analysis of the hippocampal proteome in ME7 prion disease reveals a predominant astrocytic signature and highlights the brain-restricted production of clusterin in chronic neurodegeneration
- PMID: 24366862
- PMCID: PMC3924314
- DOI: 10.1074/jbc.M113.502690
Analysis of the hippocampal proteome in ME7 prion disease reveals a predominant astrocytic signature and highlights the brain-restricted production of clusterin in chronic neurodegeneration
Abstract
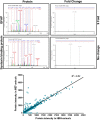
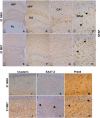
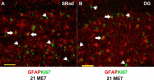
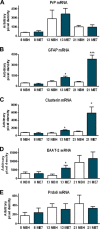
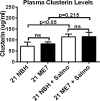
Prion diseases are characterized by accumulation of misfolded protein, gliosis, synaptic dysfunction, and ultimately neuronal loss. This sequence, mirroring key features of Alzheimer disease, is modeled well in ME7 prion disease. We used iTRAQ(TM)/mass spectrometry to compare the hippocampal proteome in control and late-stage ME7 animals. The observed changes associated with reactive glia highlighted some specific proteins that dominate the proteome in late-stage disease. Four of the up-regulated proteins (GFAP, high affinity glutamate transporter (EAAT-2), apo-J (Clusterin), and peroxiredoxin-6) are selectively expressed in astrocytes, but astrocyte proliferation does not contribute to their up-regulation. The known functional role of these proteins suggests this response acts against protein misfolding, excitotoxicity, and neurotoxic reactive oxygen species. A recent convergence of genome-wide association studies and the peripheral measurement of circulating levels of acute phase proteins have focused attention on Clusterin as a modifier of late-stage Alzheimer disease and a biomarker for advanced neurodegeneration. Since ME7 animals allow independent measurement of acute phase proteins in the brain and circulation, we extended our investigation to address whether changes in the brain proteome are detectable in blood. We found no difference in the circulating levels of Clusterin in late-stage prion disease when animals will show behavioral decline, accumulation of misfolded protein, and dramatic synaptic and neuronal loss. This does not preclude an important role of Clusterin in late-stage disease, but it cautions against the assumption that brain levels provide a surrogate peripheral measure for the progression of brain degeneration.
Keywords: Astrocytes; ME7; Mass Spectrometry (MS); Neurobiology; Neurodegeneration; Prion; Prions; iTRAQ; mRNA.
Figures



References
-
- Chiesa R., Drisaldi B., Quaglio E., Migheli A., Piccardo P., Ghetti B., Harris D. A. (2000) Accumulation of protease-resistant prion protein (PrP) and apoptosis of cerebellar granule cells in transgenic mice expressing a PrP insertional mutation. Proc. Natl. Acad. Sci. U.S.A. 97, 5574–5579 - PMC - PubMed
-
- Muchowski P. J. (2002) Protein misfolding, amyloid formation, and neurodegeneration: a critical role for molecular chaperones? Neuron 35, 9–12 - PubMed
-
- Aguzzi A. (2004) Understanding the diversity of prions. Nat. Cell Biol. 6, 290–292 - PubMed
-
- Caughey B., Baron G. S. (2006) Prions and their partners in crime. Nature 443, 803–810 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

