Flavodiiron protein Flv2/Flv4-related photoprotective mechanism dissipates excitation pressure of PSII in cooperation with phycobilisomes in Cyanobacteria
- PMID: 24367022
- PMCID: PMC3912107
- DOI: 10.1104/pp.113.231969
Flavodiiron protein Flv2/Flv4-related photoprotective mechanism dissipates excitation pressure of PSII in cooperation with phycobilisomes in Cyanobacteria
Abstract
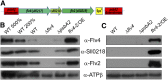
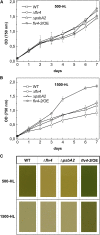
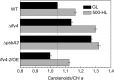
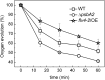
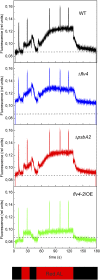
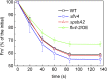
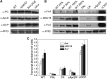
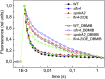
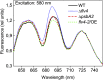
Oxygenic photosynthesis evolved with cyanobacteria, the ancestors of plant chloroplasts. The highly oxidizing chemistry of water splitting required concomitant evolution of efficient photoprotection mechanisms to safeguard the photosynthetic machinery. The role of flavodiiron proteins (FDPs), originally called A-type flavoproteins or Flvs, in this context has only recently been appreciated. Cyanobacterial FDPs constitute a specific protein group that evolved to protect oxygenic photosynthesis. There are four FDPs in Synechocystis sp. PCC 6803 (Flv1 to Flv4). Two of them, Flv2 and Flv4, are encoded by an operon together with a Sll0218 protein. Their expression, tightly regulated by CO2 levels, is also influenced by changes in light intensity. Here we describe the overexpression of the flv4-2 operon in Synechocystis sp. PCC 6803 and demonstrate that it results in improved photochemistry of PSII. The flv4-2/OE mutant is more resistant to photoinhibition of PSII and exhibits a more oxidized state of the plastoquinone pool and reduced production of singlet oxygen compared with control strains. Results of biophysical measurements indicate that the flv4-2 operon functions in an alternative electron transfer pathway from PSII, and thus alleviates PSII excitation pressure by channeling up to 30% of PSII-originated electrons. Furthermore, intact phycobilisomes are required for stable expression of the flv4-2 operon genes and for the Flv2/Flv4 heterodimer-mediated electron transfer mechanism. The latter operates in photoprotection in a complementary way with the orange carotenoid protein-related nonphotochemical quenching. Expression of the flv4-2 operon and exchange of the D1 forms in PSII centers upon light stress, on the contrary, are mutually exclusive photoprotection strategies among cyanobacteria.
Figures

Similar articles
-
Cyanobacterial Oxygenic Photosynthesis is Protected by Flavodiiron Proteins.Life (Basel). 2015 Mar 9;5(1):716-43. doi: 10.3390/life5010716. Life (Basel). 2015. PMID: 25761262 Free PMC article. Review.
-
Flavodiiron proteins in oxygenic photosynthetic organisms: photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803.PLoS One. 2009;4(4):e5331. doi: 10.1371/journal.pone.0005331. Epub 2009 Apr 24. PLoS One. 2009. PMID: 19390625 Free PMC article.
-
Operon flv4-flv2 provides cyanobacterial photosystem II with flexibility of electron transfer.Plant Cell. 2012 May;24(5):1952-71. doi: 10.1105/tpc.111.094417. Epub 2012 May 8. Plant Cell. 2012. PMID: 22570444 Free PMC article.
-
Dissecting the Photoprotective Mechanism Encoded by the flv4-2 Operon: a Distinct Contribution of Sll0218 in Photosystem II Stabilization.Plant Cell Environ. 2017 Mar;40(3):378-389. doi: 10.1111/pce.12872. Epub 2017 Jan 17. Plant Cell Environ. 2017. PMID: 27928824
-
Photoprotection in cyanobacteria: the orange carotenoid protein (OCP)-related non-photochemical-quenching mechanism.Photosynth Res. 2007 Jul-Sep;93(1-3):7-16. doi: 10.1007/s11120-007-9168-y. Epub 2007 May 8. Photosynth Res. 2007. PMID: 17486426 Review.
Cited by
-
Here comes the sun: How optimization of photosynthetic light reactions can boost crop yields.J Integr Plant Biol. 2022 Feb;64(2):564-591. doi: 10.1111/jipb.13206. J Integr Plant Biol. 2022. PMID: 34962073 Free PMC article. Review.
-
Spectrally decomposed dark-to-light transitions in Synechocystis sp. PCC 6803.Photosynth Res. 2018 Aug;137(2):307-320. doi: 10.1007/s11120-018-0505-0. Epub 2018 Mar 29. Photosynth Res. 2018. PMID: 29600442
-
Proteomic approaches in research of cyanobacterial photosynthesis.Photosynth Res. 2015 Oct;126(1):47-70. doi: 10.1007/s11120-014-0050-4. Epub 2014 Oct 31. Photosynth Res. 2015. PMID: 25359503 Review.
-
Cyanobacterial Oxygenic Photosynthesis is Protected by Flavodiiron Proteins.Life (Basel). 2015 Mar 9;5(1):716-43. doi: 10.3390/life5010716. Life (Basel). 2015. PMID: 25761262 Free PMC article. Review.
-
Synechocystis: Not Just a Plug-Bug for CO2, but a Green E. coli.Front Bioeng Biotechnol. 2014 Sep 18;2:36. doi: 10.3389/fbioe.2014.00036. eCollection 2014. Front Bioeng Biotechnol. 2014. PMID: 25279375 Free PMC article. Review.
References
-
- Adir N, Zer H, Shochat S, Ohad I. (2003) Photoinhibition - a historical perspective. Photosynth Res 76: 343–370 - PubMed
-
- Ajlani G, Vernotte C. (1998) Construction and characterization of a phycobiliprotein-less mutant of Synechocystis sp. PCC 6803. Plant Mol Biol 37: 577–580 - PubMed
-
- Allen MM. (1968) Simple conditions for growth of unicellular blue-green algae on plates. J Phycol 4: 1–4 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

