Community circulation patterns of oral polio vaccine serotypes 1, 2, and 3 after Mexican national immunization weeks
- PMID: 24367038
- PMCID: PMC4017366
- DOI: 10.1093/infdis/jit831
Community circulation patterns of oral polio vaccine serotypes 1, 2, and 3 after Mexican national immunization weeks
Abstract
Background: With wild poliovirus nearing eradication, preventing circulating vaccine-derived poliovirus (cVDPV) by understanding oral polio vaccine (OPV) community circulation is increasingly important. Mexico, where OPV is given only during biannual national immunization weeks (NIWs) but where children receive inactivated polio vaccine (IPV) as part of their primary regimen, provides a natural setting to study OPV community circulation.
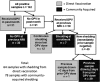
Methods: In total, 216 children and household contacts in Veracruz, Mexico, were enrolled, and monthly stool samples and questionnaires collected for 1 year; 2501 stool samples underwent RNA extraction, reverse transcription, and real-time polymerase chain reaction (PCR) to detect OPV serotypes 1, 2, and 3.
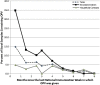
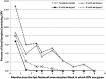
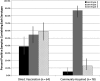
Results: OPV was detected up to 7 months after an NIW, but not at 8 months. In total, 35% of samples collected from children vaccinated the prior month, but only 4% of other samples, contained OPV. Although each serotype was detected in similar proportions among OPV strains shed as a result of direct vaccination, 87% of OPV acquired through community spread was serotype 2 (P < .0001).
Conclusions: Serotype 2 circulates longer and is transmitted more readily than serotypes 1 or 3 after NIWs in a Mexican community primarily vaccinated with IPV. This may be part of the reason why most isolated cVDPV has been serotype 2.
Keywords: Mexico; VDPV; inactivated polio vaccine; oral polio vaccine; polio.
Figures
Similar articles
-
Use of a novel real-time PCR assay to detect oral polio vaccine shedding and reversion in stool and sewage samples after a mexican national immunization day.J Clin Microbiol. 2011 May;49(5):1777-83. doi: 10.1128/JCM.02524-10. Epub 2011 Mar 16. J Clin Microbiol. 2011. PMID: 21411577 Free PMC article.
-
Protocol Paper: Oral Poliovirus Vaccine Transmissibility in Communities After Cessation of Routine Oral Poliovirus Vaccine Immunization.Clin Infect Dis. 2018 Oct 30;67(suppl_1):S115-S120. doi: 10.1093/cid/ciy606. Clin Infect Dis. 2018. PMID: 30376084 Free PMC article. Clinical Trial.
-
Real-time Polymerase Chain Reaction Analysis of Sewage Samples to Determine Oral Polio Vaccine Circulation Duration and Mutation After Mexican National Immunization Weeks.J Pediatric Infect Dis Soc. 2012 Sep;1(3):223-9. doi: 10.1093/jpids/pis062. Epub 2012 Jun 29. J Pediatric Infect Dis Soc. 2012. PMID: 23667738 Free PMC article.
-
Circulating vaccine-derived polioviruses: current state of knowledge.Bull World Health Organ. 2004 Jan;82(1):16-23. Epub 2004 Feb 26. Bull World Health Organ. 2004. PMID: 15106296 Free PMC article. Review.
-
Polio eradication: the OPV paradox.Rev Med Virol. 2003 Sep-Oct;13(5):277-91. doi: 10.1002/rmv.401. Rev Med Virol. 2003. PMID: 12931339 Review.
Cited by
-
Detection of Emerging Vaccine-Related Polioviruses by Deep Sequencing.J Clin Microbiol. 2017 Jul;55(7):2162-2171. doi: 10.1128/JCM.00144-17. Epub 2017 May 3. J Clin Microbiol. 2017. PMID: 28468861 Free PMC article.
-
The detection of 3 ambiguous type 2 vaccine-derived polioviruses (VDPV2s) in Uganda.Virol J. 2018 Apr 27;15(1):77. doi: 10.1186/s12985-018-0990-y. Virol J. 2018. PMID: 29699577 Free PMC article.
-
Deep sequencing prompts the modification of a real-time RT-PCR for the serotype-specific detection of polioviruses.J Virol Methods. 2019 Feb;264:38-43. doi: 10.1016/j.jviromet.2018.11.007. Epub 2018 Nov 14. J Virol Methods. 2019. PMID: 30447245 Free PMC article.
-
Vaccination against Clostridium difficile by Use of an Attenuated Salmonella enterica Serovar Typhimurium Vector (YS1646) Protects Mice from Lethal Challenge.Infect Immun. 2019 Jul 23;87(8):e00089-19. doi: 10.1128/IAI.00089-19. Print 2019 Aug. Infect Immun. 2019. PMID: 31138615 Free PMC article.
-
Mucosal immunity to poliovirus.Mucosal Immunol. 2022 Jan;15(1):1-9. doi: 10.1038/s41385-021-00428-0. Epub 2021 Jul 8. Mucosal Immunol. 2022. PMID: 34239028 Free PMC article. Review.
References
-
- Global Polio Eradication Initiative. Available at: http://www.polioeradication.org/Dataandmonitoring/Poliothisweek.aspx. Accessed 9 September 2013.
-
- Minor P, Macadam A, Stone D, Almond J. Genetic basis of attenuation of the Sabin oral poliovirus vaccines. Biologicals. 1993;21:357–63. - PubMed
-
- Troy S, Maldonado Y. Polioviruses. In: Long SS, Pickering LK, Prober CG, editors. Principles and practice of pediatric infectious diseases. 4th ed. China: Elsevier Inc.; 2012.
-
- (CDC) CfDCaP. Update on vaccine-derived polioviruses - worldwide, april 2011-june 2012. MMWR Morb Mortal Wkly Rep. 2012;61:741–6. - PubMed
-
- Global Polio Eradication Initiative. Available at: http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Circulat.... Accessed 20 November 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

