Distinct kinetics of synaptic structural plasticity, memory formation, and memory decay in massed and spaced learning
- PMID: 24367076
- PMCID: PMC3890840
- DOI: 10.1073/pnas.1303317110
Distinct kinetics of synaptic structural plasticity, memory formation, and memory decay in massed and spaced learning
Abstract
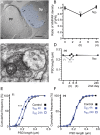
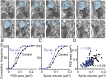
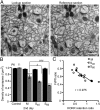
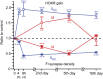
Long-lasting memories are formed when the stimulus is temporally distributed (spacing effect). However, the synaptic mechanisms underlying this robust phenomenon and the precise time course of the synaptic modifications that occur during learning remain unclear. Here we examined the adaptation of horizontal optokinetic response in mice that underwent 1 h of massed and spaced training at varying intervals. Despite similar acquisition by all training protocols, 1 h of spacing produced the highest memory retention at 24 h, which lasted for 1 mo. The distinct kinetics of memory are strongly correlated with the reduction of floccular parallel fiber-Purkinje cell synapses but not with AMPA receptor (AMPAR) number and synapse size. After the spaced training, we observed 25%, 23%, and 12% reduction in AMPAR density, synapse size, and synapse number, respectively. Four hours after the spaced training, half of the synapses and Purkinje cell spines had been eliminated, whereas AMPAR density and synapse size were recovered in remaining synapses. Surprisingly, massed training also produced long-term memory and halving of synapses; however, this occurred slowly over days, and the memory lasted for only 1 wk. This distinct kinetics of structural plasticity may serve as a basis for unique temporal profiles in the formation and decay of memory with or without intervals.
Keywords: AMPA receptor reduction; cerebellar motor learning; synapse shrinkage and elimination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- McGaugh JL. Memory—A century of consolidation. Science. 2000;287(5451):248–251. - PubMed
-
- Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294(5544):1030–1038. - PubMed
-
- Moga DE, Shapiro ML, Morrison JH. Bidirectional redistribution of AMPA but not NMDA receptors after perforant path simulation in the adult rat hippocampus in vivo. Hippocampus. 2006;16(11):990–1003. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

