Optical control of trimeric P2X receptors and acid-sensing ion channels
- PMID: 24367083
- PMCID: PMC3890850
- DOI: 10.1073/pnas.1318582111
Optical control of trimeric P2X receptors and acid-sensing ion channels
Abstract
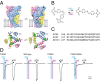
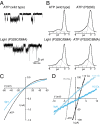
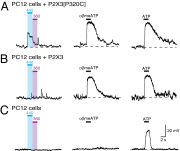
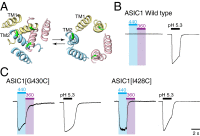
P2X receptors are trimeric membrane proteins that function as ion channels gated by extracellular ATP. We have engineered a P2X2 receptor that opens within milliseconds by irradiation at 440 nm, and rapidly closes at 360 nm. This requires bridging receptor subunits via covalent attachment of 4,4'-bis(maleimido)azobenzene to a cysteine residue (P329C) introduced into each second transmembrane domain. The cis-trans isomerization of the azobenzene pushes apart the outer ends of the transmembrane helices and opens the channel in a light-dependent manner. Light-activated channels exhibited similar unitary currents, rectification, calcium permeability, and dye uptake as P2X2 receptors activated by ATP. P2X3 receptors with an equivalent mutation (P320C) were also light sensitive after chemical modification. They showed typical rapid desensitization, and they could coassemble with native P2X2 subunits in pheochromocytoma cells to form light-activated heteromeric P2X2/3 receptors. A similar approach was used to open and close human acid-sensing ion channels (ASICs), which are also trimers but are unrelated in sequence to P2X receptors. The experiments indicate that the opening of the permeation pathway requires similar and substantial movements of the transmembrane helices in both P2X receptors and ASICs, and the method will allow precise optical control of P2X receptors or ASICs in intact tissues.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Controlling Engineered P2X Receptors with Light.Methods Mol Biol. 2020;2041:301-309. doi: 10.1007/978-1-4939-9717-6_22. Methods Mol Biol. 2020. PMID: 31646498
-
Direct gating of ATP-activated ion channels (P2X2 receptors) by lipophilic attachment at the outer end of the second transmembrane domain.J Biol Chem. 2014 Jan 10;289(2):618-26. doi: 10.1074/jbc.M113.529099. Epub 2013 Nov 22. J Biol Chem. 2014. PMID: 24273165 Free PMC article.
-
Subunit arrangement in P2X receptors.J Neurosci. 2003 Oct 1;23(26):8903-10. doi: 10.1523/JNEUROSCI.23-26-08903.2003. J Neurosci. 2003. PMID: 14523092 Free PMC article.
-
[Structual and functional dynamics of the ATP receptor channel P2X(2)].Brain Nerve. 2010 Dec;62(12):1323-9. Brain Nerve. 2010. PMID: 21139185 Review. Japanese.
-
Bridging the gap between structural bioinformatics and receptor research: the membrane-embedded, ligand-gated, P2X glycoprotein receptor.Curr Top Med Chem. 2004;4(16):1657-705. doi: 10.2174/1568026043387197. Curr Top Med Chem. 2004. PMID: 15579102 Review.
Cited by
-
Manipulation of P2X Receptor Activities by Light Stimulation.Mediators Inflamm. 2016;2016:7852168. doi: 10.1155/2016/7852168. Epub 2016 Jan 17. Mediators Inflamm. 2016. PMID: 26884649 Free PMC article. Review.
-
Exploring conformational states and helical packings in the P2X receptor transmembrane domain by molecular dynamics simulation.J Biol Phys. 2018 Sep;44(3):331-344. doi: 10.1007/s10867-018-9493-8. Epub 2018 Apr 2. J Biol Phys. 2018. PMID: 29611030 Free PMC article.
-
Proton and non-proton activation of ASIC channels.PLoS One. 2017 Apr 6;12(4):e0175293. doi: 10.1371/journal.pone.0175293. eCollection 2017. PLoS One. 2017. PMID: 28384246 Free PMC article.
-
Origin and de novo domestication of sweet orange.Nat Genet. 2025 Mar;57(3):754-762. doi: 10.1038/s41588-025-02122-4. Epub 2025 Mar 5. Nat Genet. 2025. PMID: 40045092 Free PMC article.
-
Optical control of PIEZO1 channels.Nat Commun. 2023 Mar 7;14(1):1269. doi: 10.1038/s41467-023-36931-0. Nat Commun. 2023. PMID: 36882406 Free PMC article.
References
-
- Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442(7102):527–532. - PubMed
-
- Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333–359. - PubMed
-
- Krishtal OA, Pidoplichko VI. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

