Physiology and pathophysiology of prostanoid receptors
- PMID: 24367153
- PMCID: PMC3859365
- DOI: 10.2183/pjab/83.296
Physiology and pathophysiology of prostanoid receptors
Abstract
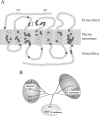
Prostanoids, consisting of prostaglandins (PGs) and thromboxanes (TXs), are oxygenated products of C20 unsaturated fatty acids. They include PGD2, PGE2, PGF2 α , PGI2, and TXA2. Given that aspirin-like nonsteroidal anti-inflammatory drugs exert their actions by suppressing prostanoid production, prostanoids have been implicated in processes inhibited by these drugs, including inflammation, fever, and pain. Prostanoids also contribute to vascular homeostasis, reproduction, and regulation of kidney and gastrointestinal functions. How prostanoids exert such a variety of actions had remained unclear, however. Prostanoids are released outside of cells immediately after their synthesis and exert their actions by binding to receptors on target cells. We have identified a family of eight types or subtypes of G protein-coupled receptors that mediate prostanoid actions. Another G protein-coupled receptor was also identified as an additional receptor for PGD2. Genes for these receptors have been individually disrupted in mice, and analyses of these knockout mice have not only elucidated the molecular and cellular mechanisms of known prostanoid actions but also revealed previously unknown actions. In this article, I review the physiological and pathophysiological roles of prostanoids and their receptors revealed by these studies.
Keywords: G protein–coupled receptor; cyclooxygenase; prostaglandin; thromboxane.
Figures








References
Publication types
LinkOut - more resources
Full Text Sources
