p130Cas substrate domain signaling promotes migration, invasion, and survival of estrogen receptor-negative breast cancer cells
- PMID: 24367162
- PMCID: PMC3022348
- DOI: 10.2147/bctt.s6255
p130Cas substrate domain signaling promotes migration, invasion, and survival of estrogen receptor-negative breast cancer cells
Abstract
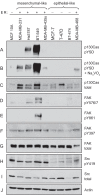
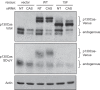
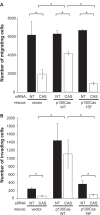
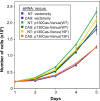
Elevated Src tyrosine kinase activity is commonly observed in breast cancer and likely contributes to neoplasia and malignancy. p130Cas ("Crk-associated substrate") is a major Src substrate found at the sites where integrins mediate cell adhesion to the extracellular matrix. Src phosphorylates multiple tyrosines in the p130Cas "substrate domain" (SD) and this signaling event has been implicated in the promotion of cell motility, primarily from studies on fibroblasts. In breast cancer, studies on p130Cas have focused on its role in conferring antiestrogen resistance to cells that express the estrogen receptor (ER+). However, little is known regarding the role of p130Cas in the more aggressive estrogen receptor negative (ER-) breast cancers for which there is a need for development of effective targeted therapies. We found high levels of p130Cas SD tyrosine phosphorylation to be a common characteristic of ER- breast cancer cell lines, with particularly high levels observed for the BT-549 cell line. Using RNA interference to knock down p130Cas expression in BT-549 cells, combined with rescue by WT p130Cas versus a signaling-deficient control, we provide evidence that p130Cas SD tyrosine phosphorylation is an important signaling event in the migration, invasion, proliferation, and survival of this ER-breast cancer cell line.
Keywords: BCAR1; FAK; Src; adhesion; integrins; tyrosine phosphorylation.
Figures



References
-
- Frame MC. Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta. 2002;1602(2):114–130. - PubMed
-
- Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22(4):337–358. - PubMed
-
- Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23(48):7928–7946. - PubMed
-
- Mayer BJ, Hamaguchi M, Hanafusa H. A novel viral oncogene with structural similarity to phospholipase C. Nature. 1988;332(6161):272–275. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

