Persistence of immunity from 1 year of age after one or two doses of hepatitis A vaccine given to children in Argentina
- PMID: 24367232
- PMCID: PMC3846818
- DOI: 10.2147/HMER.S33847
Persistence of immunity from 1 year of age after one or two doses of hepatitis A vaccine given to children in Argentina
Abstract
Background: This study was done to determine the immunogenicity of a single dose of hepatitis A vaccine in children, providing needed clinical data on the flexibility of booster administration.
Methods: Participants had received one dose of inactivated hepatitis A vaccine (Avaxim™ 80 U Pediatric) at 12-23 months of age or two doses of the same vaccine at 12 and 18 months of age prior to enrolment. Anti-hepatitis A antibody concentrations were measured at the first, second, and third year after vaccination. Suspected cases of hepatitis A in participant families were assessed and family socioeconomic data were collected.
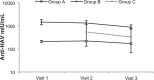
Results: A series of 546 participants were enrolled. Of 467 (85.5%) participants completing 3 years of follow-up, 365 had received a single vaccine dose and 94 had received two vaccine doses. Seropositivity (anti-HAV ≥ 10 mIU/mL) at 3 years was 99.7% after one dose and 100% after two doses. At one year, geometric mean concentrations were higher after two doses (1433.9 mIU/mL, 95% confidence interval [CI] 1108-1855) than one (209.7 mIU/mL, 95% CI 190.6-230.6). Geometric mean concentrations decreased in both groups during the study, but remained well above 10 mIU/mL through the third year. The geometric mean of 3-year to one-year anti-hepatitis A concentration ratios was 0.74 (95% CI 0.70-0.79) following one dose and 0.57 (95% CI 0.47-0.70) following two doses. The greatest decrease in geometric mean concentrations occurred during the third year, ie, 21.2% in the one-dose group and 40.8% in the two-dose group. Six participants became seronegative during follow-up and responded strongly to a booster dose. Anti-hepatitis A concentrations increased in 135 children (34.9%) in the second year and 50 (13.7%) in the third year; none lived in a family with a case of hepatitis A. Three confirmed cases of hepatitis A occurred in family members. Participants belonged to a middle-income, urban/suburban population with good sanitation facilities and water supplies.
Conclusion: A single dose of hepatitis A vaccine at 12-23 months of age resulted in hepatitis A seropositivity in all but one vaccinee after 3 years. Increased anti-hepatitis A serum concentrations suggested exposure to wild-type hepatitis A virus in this middle-class socioeconomic environment. Continuing surveillance is required to confirm the effectiveness of a single-dose hepatitis A vaccination; however, the results of the first three years are encouraging.
Keywords: antibody persistence; hepatitis A vaccine; immunization programs; single dose.
Figures

References
-
- World Health Organization Hepatitis A vaccines. Wkly Epidemiol Rec. 2000;75(5):38–44. - PubMed
-
- Tapia-Conyer R, Santos JI, Cavalcanti AM, et al. Hepatitis A in Latin America: a changing epidemiologic pattern. Am J Trop Med Hyg. 1999;61(5):825–829. - PubMed
-
- Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28(41):6653–6657. - PubMed
-
- Gonzalez J, Fay O, Canero-Velasco MC, et al. Hepatitis A virus infection in children in Argentina: a pilot study. Acta Gastroenterol Latinoam. 1997;27(5):331–334. Spanish. - PubMed
-
- Vacchino MN. Incidence of hepatitis A in Argentina after vaccination. J Viral Hepat. 2008;15(Suppl 2):47–50. - PubMed
LinkOut - more resources
Full Text Sources

