Target-cell-specific short-term plasticity in local circuits
- PMID: 24367330
- PMCID: PMC3854841
- DOI: 10.3389/fnsyn.2013.00011
Target-cell-specific short-term plasticity in local circuits
Abstract
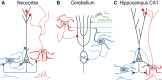
Short-term plasticity (STP) denotes changes in synaptic strength that last up to tens of seconds. It is generally thought that STP impacts information transfer across synaptic connections and may thereby provide neurons with, for example, the ability to detect input coherence, to maintain stability and to promote synchronization. STP is due to a combination of mechanisms, including vesicle depletion and calcium accumulation in synaptic terminals. Different forms of STP exist, depending on many factors, including synapse type. Recent evidence shows that synapse dependence holds true even for connections that originate from a single presynaptic cell, which implies that postsynaptic target cell type can determine synaptic short-term dynamics. This arrangement is surprising, since STP itself is chiefly due to presynaptic mechanisms. Target-specific synaptic dynamics in addition imply that STP is not a bug resulting from synapses fatiguing when driven too hard, but rather a feature that is selectively implemented in the brain for specific functional purposes. As an example, target-specific STP results in sequential somatic and dendritic inhibition in neocortical and hippocampal excitatory cells during high-frequency firing. Recent studies also show that the Elfn1 gene specifically controls STP at some synapse types. In addition, presynaptic NMDA receptors have been implicated in synapse-specific control of synaptic dynamics during high-frequency activity. We argue that synapse-specific STP deserves considerable further study, both experimentally and theoretically, since its function is not well known. We propose that synapse-specific STP has to be understood in the context of the local circuit, which requires combining different scientific disciplines ranging from molecular biology through electrophysiology to computer modeling.
Keywords: development; network models; short-term plasticity; synapse formation; synapse specificity; synaptic disease.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

