High throughput nanostructure-initiator mass spectrometry screening of microbial growth conditions for maximal β-glucosidase production
- PMID: 24367356
- PMCID: PMC3854461
- DOI: 10.3389/fmicb.2013.00365
High throughput nanostructure-initiator mass spectrometry screening of microbial growth conditions for maximal β-glucosidase production
Abstract
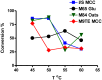
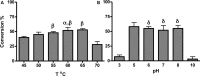
Production of biofuels via enzymatic hydrolysis of complex plant polysaccharides is a subject of intense global interest. Microbial communities are known to express a wide range of enzymes necessary for the saccharification of lignocellulosic feedstocks and serve as a powerful reservoir for enzyme discovery. However, the growth temperature and conditions that yield high cellulase activity vary widely, and the throughput to identify optimal conditions has been limited by the slow handling and conventional analysis. A rapid method that uses small volumes of isolate culture to resolve specific enzyme activity is needed. In this work, a high throughput nanostructure-initiator mass spectrometry (NIMS)-based approach was developed for screening a thermophilic cellulolytic actinomycete, Thermobispora bispora, for β-glucosidase production under various growth conditions. Media that produced high β-glucosidase activity were found to be I/S + glucose or microcrystalline cellulose (MCC), Medium 84 + rolled oats, and M9TE + MCC at 45°C. Supernatants of cell cultures grown in M9TE + 1% MCC cleaved 2.5 times more substrate at 45°C than at all other temperatures. While T. bispora is reported to grow optimally at 60°C in Medium 84 + rolled oats and M9TE + 1% MCC, approximately 40% more conversion was observed at 45°C. This high throughput NIMS approach may provide an important tool in discovery and characterization of enzymes from environmental microbes for industrial and biofuel applications.
Keywords: NIMS; enzymatic activity screening; high throughput; microbial communities; β-glucosidase.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

