Distinct iPS Cells Show Different Cardiac Differentiation Efficiency
- PMID: 24367382
- PMCID: PMC3842496
- DOI: 10.1155/2013/659739
Distinct iPS Cells Show Different Cardiac Differentiation Efficiency
Abstract
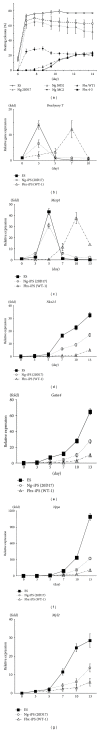
Patient-specific induced pluripotent stem (iPS) cells can be generated by introducing transcription factors that are highly expressed in embryonic stem (ES) cells into somatic cells. This opens up new possibilities for cell transplantation-based regenerative medicine by overcoming the ethical issues and immunological problems associated with ES cells. Despite the development of various methods for the generation of iPS cells that have resulted in increased efficiency, safety, and general versatility, it remains unknown which types of iPS cells are suitable for clinical use. Therefore, the aims of the present study were to assess (1) the differentiation potential, time course, and efficiency of different types of iPS cell lines to differentiate into cardiomyocytes in vitro and (2) the properties of the iPS cell-derived cardiomyocytes. We found that high-quality iPS cells exhibited better cardiomyocyte differentiation in terms of the time course and efficiency of differentiation than low-quality iPS cells, which hardly ever differentiated into cardiomyocytes. Because of the different properties of the various iPS cell lines such as cardiac differentiation efficiency and potential safety hazards, newly established iPS cell lines must be characterized prior to their use in cardiac regenerative medicine.
Figures





Similar articles
-
Generation of functional murine cardiac myocytes from induced pluripotent stem cells.Circulation. 2008 Jul 29;118(5):507-17. doi: 10.1161/CIRCULATIONAHA.108.778795. Epub 2008 Jul 14. Circulation. 2008. PMID: 18625890
-
iPS cells: a source of cardiac regeneration.J Mol Cell Cardiol. 2011 Feb;50(2):327-32. doi: 10.1016/j.yjmcc.2010.10.026. Epub 2010 Oct 30. J Mol Cell Cardiol. 2011. PMID: 21040726 Review.
-
Induced pluripotent stem cells in cardiovascular medicine.Stem Cells Int. 2011;2011:348960. doi: 10.4061/2011/348960. Epub 2011 Oct 2. Stem Cells Int. 2011. PMID: 21977041 Free PMC article.
-
Generation of functional cardiomyocytes from mouse induced pluripotent stem cells.Int J Cardiol. 2011 Dec 15;153(3):277-85. doi: 10.1016/j.ijcard.2010.08.052. Epub 2010 Sep 25. Int J Cardiol. 2011. PMID: 20870305
-
[Review of basic studies about the cardiac stem cell and regenerative medicine].Nihon Rinsho. 2008 May;66(5):908-14. Nihon Rinsho. 2008. PMID: 18464509 Review. Japanese.
Cited by
-
Robust Generation of Cardiomyocytes from Human iPS Cells Requires Precise Modulation of BMP and WNT Signaling.Stem Cell Rev Rep. 2015 Aug;11(4):560-9. doi: 10.1007/s12015-014-9564-6. Stem Cell Rev Rep. 2015. PMID: 25392050 Free PMC article.
-
Are These Cardiomyocytes? Protocol Development Reveals Impact of Sample Preparation on the Accuracy of Identifying Cardiomyocytes by Flow Cytometry.Stem Cell Reports. 2019 Feb 12;12(2):395-410. doi: 10.1016/j.stemcr.2018.12.016. Epub 2019 Jan 24. Stem Cell Reports. 2019. PMID: 30686762 Free PMC article.
-
iPS cell-derived cardiogenicity is hindered by sustained integration of reprogramming transgenes.Circ Cardiovasc Genet. 2014 Oct;7(5):667-76. doi: 10.1161/CIRCGENETICS.113.000298. Epub 2014 Jul 30. Circ Cardiovasc Genet. 2014. PMID: 25077947 Free PMC article.
-
Evaluation of Pancreatic β-cell Differentiation Efficiency of Human iPSC Lines for Clinical Use.Curr Stem Cell Res Ther. 2024;19(11):1449-1460. doi: 10.2174/011574888X267226231126185532. Curr Stem Cell Res Ther. 2024. PMID: 38311917
-
Action potential variability in human pluripotent stem cell-derived cardiomyocytes obtained from healthy donors.Front Physiol. 2022 Dec 16;13:1077069. doi: 10.3389/fphys.2022.1077069. eCollection 2022. Front Physiol. 2022. PMID: 36589430 Free PMC article.
References
-
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–156. - PubMed
-
- Thomson JA. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. - PubMed
-
- Yuasa S, Itabashi Y, Koshimizu U, et al. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nature Biotechnology. 2005;23:607–611. - PubMed
-
- Shimoji K, Yuasa S, Onizuka T, et al. G-CSF promotes the proliferation of developing cardiomyocytes in vivo and in derivation from ESCs and iPSCs. Cell Stem Cell. 2010;6(3):227–237. - PubMed
-
- Onizuka T, Yuasa S, Kusumoto D, et al. Wnt2 accelerates cardiac myocyte differentiation from ES-cell derived mesodermal cells via non-canonical pathway. Journal of Molecular and Cellular Cardiology. 2012;52(3):650–659. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

