Recent advances in transition metal-catalyzed Csp(2)-monofluoro-, difluoro-, perfluoromethylation and trifluoromethylthiolation
- PMID: 24367416
- PMCID: PMC3869273
- DOI: 10.3762/bjoc.9.287
Recent advances in transition metal-catalyzed Csp(2)-monofluoro-, difluoro-, perfluoromethylation and trifluoromethylthiolation
Abstract
In the last few years, transition metal-mediated reactions have joined the toolbox of chemists working in the field of fluorination for Life-Science oriented research. The successful execution of transition metal-catalyzed carbon-fluorine bond formation has become a landmark achievement in fluorine chemistry. This rapidly growing research field has been the subject of some excellent reviews. Our approach focuses exclusively on transition metal-catalyzed reactions that allow the introduction of -CFH2, -CF2H, -C n F2 n +1 and -SCF3 groups onto sp² carbon atoms. Transformations are discussed according to the reaction-type and the metal employed. The review will not extend to conventional non-transition metal methods to these fluorinated groups.
Keywords: catalysis; cross-coupling; difluoromethylation; fluorine; monofluoromethylation; organo-fluorine; transition metal; trifluoromethylation; trifluoromethylthiolation.
Figures
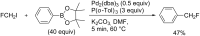
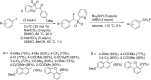

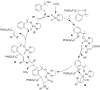
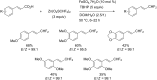
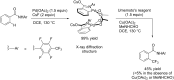
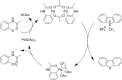
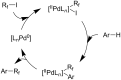
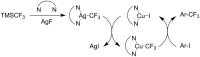
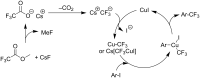
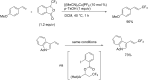
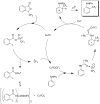
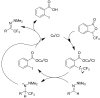
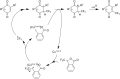
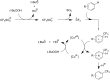
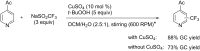
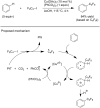
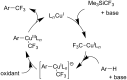

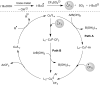
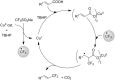

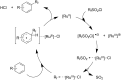
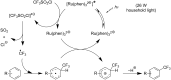
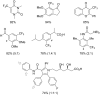
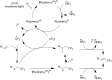
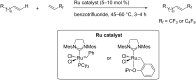
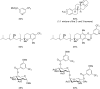
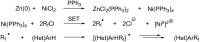
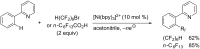
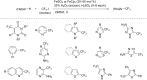
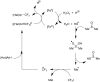
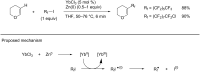
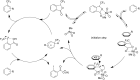
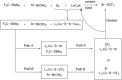

References
-
- Banks R E, Smart B E, Tatlow J C, editors. Organofluorine Chemistry: Principles and Commercial Applications. New York: Plenum Press; 2000.
-
- Hiyama T, Kanie K, Kusumoto T, et al. Organofluorine Compounds: Chemistry and Application. Berlin: Springer-Verlag; 2000.
-
- Kirsch P. Modern Fluoroorganic Chemistry. Weinheim, Germany: Wiley-VCH; 2004. - DOI
-
- Chambers R D. Fluorine in Organic Chemistry. Oxford, U.K.: Blackwell; 2004.
-
- Uneyama K. Organofluorine Chemistry. Oxford, U.K.: Blackwell; 2006. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
