Multigramme synthesis and asymmetric dihydroxylation of a 4-fluorobut-2E-enoate
- PMID: 24367430
- PMCID: PMC3869297
- DOI: 10.3762/bjoc.9.301
Multigramme synthesis and asymmetric dihydroxylation of a 4-fluorobut-2E-enoate
Abstract
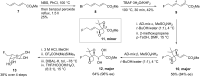
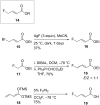
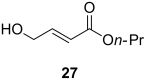
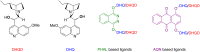
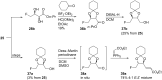
Esters of crotonic acid were brominated on a multigramme scale using a free radical procedure. A phase transfer catalysed fluorination transformed these species to the 4-fluorobut-2E-enoates reproducibly and at scale (48-53%, ca. 300 mmol). Asymmetric dihydroxylation reactions were then used to transform the butenoate, ultimately into all four diastereoisomers of a versatile fluorinated C4 building block at high enantiomeric-enrichment. The (DHQ)2AQN and (DHQD)2AQN ligands described by Sharpless were the most effective. The development and optimisation of a new and facile method for the determination of ee is also described; (19)F{(1)H} spectra recorded in d-chloroform/diisopropyl tartrate showed distinct baseline separated signals for different enantiomers.
Keywords: asymmetric; dihydroxylation; ee determination; fluorination; fluorosugars; organo-fluorine.
Figures







Similar articles
-
Developing an asymmetric, stereodivergent route to selected 6-deoxy-6-fluoro-hexoses.Org Biomol Chem. 2009 Mar 7;7(5):996-1008. doi: 10.1039/b815342f. Epub 2009 Jan 23. Org Biomol Chem. 2009. PMID: 19225683
-
Silver(I)-directed growth of metal-organic complex nanocrystals with bidentate ligands of hydroquinine anthraquinone-1,4-diyl diethers as linkers at the water-chloroform interface.Nanoscale Res Lett. 2014 Sep 12;9(1):488. doi: 10.1186/1556-276X-9-488. eCollection 2014. Nanoscale Res Lett. 2014. PMID: 25246874 Free PMC article.
-
An electrophilic cleavage procedure for the asymmetric dihydroxylation: direct enantioselective synthesis of cyclic boronic esters from olefins.Chemistry. 2005 Jun 20;11(13):3951-8. doi: 10.1002/chem.200500095. Chemistry. 2005. PMID: 15844136
-
Sharpless Asymmetric Dihydroxylation: An Impressive Gadget for the Synthesis of Natural Products: A Review.Molecules. 2023 Mar 17;28(6):2722. doi: 10.3390/molecules28062722. Molecules. 2023. PMID: 36985698 Free PMC article. Review.
-
Stereoselective Synthesis of Flavonoids: A Brief Overview.Molecules. 2023 Jan 3;28(1):426. doi: 10.3390/molecules28010426. Molecules. 2023. PMID: 36615614 Free PMC article. Review.
References
-
- Withers S G, MacLennan D J, Street I P. Carbohydr Res. 1986;154:127. doi: 10.1016/S0008-6215(00)90028-4. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
